Abstract
Objective
To study antimicrobial use for benchmarking and ensuring quality of antimicrobial treatment and to identify risk factors associated with the high use of antimicrobials in German intensive care units (ICUs) through implementation of the SARI (Surveillance of Antimicrobial Use and Antimicrobial Resistance in ICUs) system.
Design
Prospective, unit-based surveillance on antimicrobial use from February, 2000, until June, 2002. The data are standardised by use of the defined daily dose (DDD) for each antimicrobial defined by the WHO and by calculating use per 1000 patient days.
Setting
The data were obtained from 35 German ICUs and stratified by type of ICU (medical, surgical, interdisciplinary).
Results
To date, the project covers a total of 266,013 patient days in 744 reported ICU months and 354,356 DDDs. Mean antimicrobial use density (AD) was 1,332 DDD/1000 patient days and was correlated with length of stay. Penicillins with beta-lactamase inhibitor (AD 338.3) and quinolones (155.5) were the antimicrobial group with the highest ADs. Comparison with US ICARE (Intensive Care Antimicrobial Resistance Epidemiology)/AUR (Antimicrobial Use and Resistance) data revealed a higher AD for glycopeptides and 3rd generation cephalosporins in ICARE/AUR ICUs, but a higher AD for carbapenems in German SARI ICUs regardless of the type of ICU. In the multivariate analysis, length of stay was an independent risk factor for an AD above the 75% percentile of the total amount of antimicrobials used (OR 1.96 per day); likewise, for the AD above the 75% percentile of carbapenems (OR 1.90 per day) and penicillins with extended spectrum (OR 2.01 per day). High use of glycopeptides and quinolones (AD >75% percentile) correlated with central venous catheter (CVC) rate (OR 1.14 per CVC day per 100 patient days and 1.16, respectively).
Conclusion
The SARI data on antimicrobials serve ICUs as a benchmark by which to improve the quality of antimicrobial drug administration and for international comparison.
Similar content being viewed by others
Introduction
The European Commission and the World Health Organisation (WHO) have recognised the importance of prudence in the use of antimicrobial agents [1]. Beside the contribution of antimicrobials to the treatment of infectious diseases, antimicrobial use has been accompanied by an increasing prevalence of micro-organisms resistant to one or more antimicrobials [2]. Inappropriate use of antimicrobials and antimicrobial resistance pose a threat to public health, are costly and have economic and ecological implications for society [3].
The intensive care setting is of principal interest because of the frequent and extended use of antimicrobials and invasive devices, the acute or chronic suppression of the immune system in ICU patients, the higher nursing index compared to non-ICU areas and the increased likelihood of cross transmission of resistant pathogens. This makes ICUs a high-risk area for the selection and spread of antimicrobial-resistant bacteria [4]. Although many possible methods have been proposed to reduce inappropriate antimicrobial use, it may be difficult to decide which one will prove successful in a particular setting [5]. Comparison of data as a benchmarking instrument is an important initial step, and serves to help participating ICUs recognise problems and improve antimicrobial use.
The SARI project is supported by the German Ministry of Science and Education (BMBF). The main objective of the project is to provide information on the use of antimicrobials in a subset of KISS (Krankenhaus = hospital infection surveillance system) hospitals and on the percentages of resistant bacterial pathogens isolated in the microbiology laboratories of these hospitals. KISS provides reference data on hospital infections throughout Germany (www.nrz-hygiene.de). ICU-specific, national reference data concerning both these questions have been collected. Project SARI attempts to investigate the relationship between antimicrobial use and the resistance of the most common organisms causing nosocomial infections.
This work reports the first results on the surveillance of antimicrobial use and antimicrobial resistance in intensive care units (SARI), which is the first of its kind in Europe. This paper presents and analyses the antimicrobial use data of 35 German ICUs over a period of more than 2 years (2/2000–6/2002). Data on antimicrobial resistance and the relationship between antimicrobial use and resistance will be published in a second paper.
Methods
Participating hospitals
Hospitals participating in the ICU surveillance component of the KISS ICU system were invited to join the SARI project. In order to provide data on ICU-associated infections, KISS, the German nosocomial infections surveillance system, was established in 1997, using a surveillance protocol based on the national nosocomial infections surveillance (NNIS) system. The ICU surveillance component focuses on lower respiratory tract infections (pneumonia and bronchitis), blood stream and urinary tract infections in intensive care units. National reference data are generated for device-associated infection rates. This surveillance component is unit-based. Until 12/2002 KISS included 274 ICUs and 2,145,793 patient days. The methods employed in the projects KISS and SARI are described in greater detail elsewhere [6, 7].
Pharmacy data
Participating ICUs reported oral and parenteral antimicrobials in grams monthly. The quantity of antimicrobial drugs was standardised by conversion to defined daily doses (DDDs) according to the ATC classification employed by the WHO [8]. All the antimicrobial classes (see corresponding ATC codes in the ESM) and all antimicrobials used by the participants were surveyed. Details are provided in the appendix for ATC codes. A DDD is defined as the assumed average maintenance dose per day for a drug used for its main indication in adults. It should be emphasised that the DDD, especially in ICUs, is a unit of measurement and does not necessarily reflect the recommended or prescribed daily dose. To control the population size at risk of receiving antimicrobials, we determined the antimicrobial use density (AD), expressed as DDD per 1000 patient days for each antimicrobial agent. To derive the number of DDDs per 1000 patient days, the pooled number of grams of each antimicrobial applied in the ICUs per month was divided by the number of grams per DDD for a specified antimicrobial; this figure was then divided by the number of patient days in the respective ICU and multiplied by 1000.
In addition, the total AD of all antimicrobials in WHO ATC group J01 used by the participating ICUs and ADs of antimicrobial groups were calculated. Participating ICUs were stratified by type of hospital (university, affiliation to medical school and others) and by type of ICU (interdisciplinary, surgical and medical). Statistical data on antimicrobial use was fed back on a quarterly basis to participating ICUs.
Surveillance data on nosocomial infections
Data on patient characteristics were obtained from the KISS system, i.e. device-associated nosocomial infection rates (pneumonia, blood stream infection and urinary tract infection), device days and mean length of stay. The incidence density of nosocomial infections was calculated by relating the number of device-associated nosocomial infections to 1000 patient days or 1000 device days, respectively. KISS focuses on the most important ICU infections (in the application of Centers for Disease Control and Prevention [CDC] definitions of nosocomial infections [9]): ventilator-associated pneumonia; central venous catheter-associated bloodstream infection and catheter-associated urinary tract infection.
Statistical analysis
The pharmacy data were analysed quarterly by using SAS version 8.01 software (SAS). The key percentile distributions of ADs were calculated and pooled over time. As the distribution of ADs was generally skewed, we applied non-parametric tests. For the analysis of ADs by type of ICU, differences between the antimicrobial groups were tested with the Wilcoxon-W test for two samples (e.g. surgical versus non-surgical). To evaluate the change in ADs over time, quarterly ADs were adapted to a linear regression model for all ICUs together and individually by ICU type.
To identify risk factors for high use density, univariate and multivariate analyses were carried out. In the univariate analysis we determined Spearman-correlation for continuous variables. For significant correlation we generated scatter plots with linear trends. Differences between the categories (e.g. university versus non-university) were tested using the Wilcoxon-W test. In the multivariate analysis we performed the logistic regression model by forward stepwise selection. The value of the 75th percentile of antimicrobial use density was used to differentiate between high and less high use density in the logistic regression model. The following risk factors were analysed: size of hospital (≤200, 201–400, 401–600, >600 beds) and, as continuous variables, number of ICU beds, incidence density of nosocomial infections (number of nosocomial infections per 1000 patient days), urinary catheter-associated urinary tract infection rate (number of catheter-related urinary tract infections per 1000 urinary catheter days), central line-associated blood stream infection rate (number of central line-associated blood stream infections per 1000 central line days), ventilator-associated pneumonia rate (number of ventilator-associated pneumonia per 1000 ventilation days), length of stay (days), central venous catheter utilisation (central line days per 100 patient days), ventilator utilisation rate (ventilator days per 100 patient days) and urinary catheter utilisation rate (number of urinary catheter days per 100 patient days).
To compare antimicrobial use data between SARI and ICARE/AUR, data that are widely published, the SARI data were calculated according to the antimicrobial group classification and DDDs used by ICARE/AUR [10]. Differences between antimicrobial use density in project ICARE/AUR and SARI were tested by one-sample Wilcoxon rank sum test. The significance level was set at an alpha of 0.05.
Results
Participating hospitals
Surveillance of antimicrobial use and antimicrobial resistance in intensive care units (SARI) started in 2/2000 with 12 ICUs. By 2001 the number had increased to 35 ICUs. Twenty KISS hospitals representing 35 ICUs submitted data to SARI and the ICU component of the KISS system. The 35 ICUs in 17 geographically separated locations represent different categories of number of beds and teaching affiliation: thirteen of these belong to university hospitals, 16 are affiliated to a medical school and 6 ICUs are located in other hospitals. Twenty-six ICUs are located in hospitals with more than 600 beds; only one is in a hospital with fewer than 200 beds. The number of beds per ICU ranges from 6 to 26, and almost half of the ICUs (17) have between 9 and 14 beds. Fourteen of the ICUs are interdisciplinary, 11 are surgical or neurosurgical and 10 are medical. All of them reported data for at least 13 months (total number of months of participation: 744, ranging from 13 to 29 months).
Antimicrobial use density
The data on antimicrobial use were collected from 35 ICUs covering 354,356 DDDs during 744 reported ICU months and 266,013 patient days. Table 1 provides data on the number of DDDs, the mean antimicrobial ADs and key percentiles (25th, median and 75th). The mean AD was 1,332 DDDs/1000 patient days during the period 2/2000–6/2002 (median AD 1300). Pooled antimicrobial ADs for all ICUs revealed the highest ADs for penicillins with beta-lactamase inhibitors followed by quinolones, second generation cephalosporins, third generation cephalosporins and carbapenems (Table 1).The most frequently applied single antimicrobial agent was amoxicillin/clavulanic acid (number of DDDs 55,216, AD 208), followed by parenteral ciprofloxacin (number of DDDs 27,579, AD 104), parenteral cefuroxime (number of DDDs 24,264, AD 91), ampicillin-sulbactam (number of DDDs 19,109, AD 71.8), erythromycin (number of DDDs 15,874, AD 59.7), metronidazole (number of DDDs 13,500, AD 50.8) and imipenem (number of DDDs 13,132, AD 49.4).
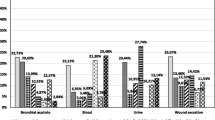
In Fig.1, the median antimicrobial ADs were stratified according to type of ICU. The median of total antimicrobial use was 1,252 for interdisciplinary ICUs, 1,386 for surgical ICUs and 1,483 for medical ICUs. Although the preferred antimicrobial group was penicillins with lactamase inhibitor followed by quinolones in all types of ICU, other differences were found among the different types of ICUs. Carbapenems (mean AD 107, median AD 113) and glycopeptides (mean AD 57, median AD 36) were used almost twice as frequently in surgical ICUs compared with interdisciplinary ICUs (mean AD 64, median AD 51 and mean AD 30, median AD 22, respectively), whereas medical ICUs had an at least four-fold higher AD for macrolides (mean AD 151, median AD 89) than surgical ICUs (mean AD 34, median AD 30). ADs differed significantly by type of ICU for glycopeptides (interdisciplinary/non-interdisciplinary ICUs, p=0.015), macrolides (medical/non-medical ICUs, p=0.033; surgical/non-surgical ICUs, p=0.017) and penicillins with extended spectrum (surgical/non-surgical ICUs, p=0.022).
From 2/2000 to 6/2002 a significant increase was observed in the AD of penicillins with extended spectrum (p<0.001) and a significant decrease in the AD of 2nd generation cephalosporins (p=0.02) for all types of ICUs. The application of glycopeptides (p=0.042), carbapenems (p=0.048), penicillins with beta-lactamase inhibitor (p=0.002), quinolones (p=0.033) and macrolides (p=0.01) also decreased significantly in interdisciplinary ICUs.
During the observation period, total antimicrobial use was significantly correlated with mean length of stay in the intensive care unit (Fig. 2). No difference in the overall use density could be shown by stratifying according to ICU teaching affiliation (university versus non-university) or according to the number of hospital beds (fewer or more than 600 beds). However, ICUs in hospitals with more than 600 beds had a significantly higher carbapenem and glycopeptide use. Furthermore, there was a positive correlation between the ventilator utilisation rate and the use of quinolones, whereas there was a negative correlation between the rate of ventilator-associated pneumonia and the use of quinolones.
The univariate analysis did not show a relationship between total antimicrobial AD and the recorded rate of nosocomial infections in the ICUs (Table 2). The device-associated nosocomial infection rate was correlated with mean length of stay (correlation coefficient 0.34, p=0.045).
In the multivariate analysis, the parameters independently associated with an antimicrobial use density higher than 75% percentile of all participants are shown in Table 3. The data on mean and median antimicrobial usage density of different antimicrobial groups and agents of German SARI ICUs and US American ICARE/AUR ICUs are provided in Table 4. Because Project ICARE/AUR did not report antimicrobial use in WHO DDDs/1000 patient days, the AD for SARI ICUs were recalculated in ICARE/AUR DDDs/1000 patient days.
Discussion
We present the first data collected by SARI on antimicrobial use in German ICUs. These may serve as reference data and can be of service in understanding the relationship between antimicrobial use and the emergence of resistance.
Only one out of the 35 ICUs providing data is located in a hospital with fewer than 200 beds, but the total antimicrobial AD did not differ significantly by size of hospital or type of ICU. However, the ADs of antimicrobial groups varied by type of ICU. To take different patient populations into account we stratified by type of ICU, in a similar way to ICARE/AUR [11]. It was not surprising that higher macrolide ADs were found in medical, rather than surgical ICUs, from which it may be hypothesised that more patients with atypical pneumonia, for instance, are treated there. In accordance with data from ICARE/AUR, surgical ICUs used twice as many glycopeptides as interdisciplinary ICUs. In a recent study of KISS hospitals, surgical German ICUs were shown to be a risk factor for methicillin-resistant Staphylococcus aureus (MRSA) [12]). In the ICARE/AUR project, resistance rates were combined for all ICU types because detailed analysis demonstrated that resistance rates generally do not differ according to the type of ICU [13].
The median total antimicrobial AD was 1,300 (mean AD 1,332) in all SARI ICUs combined, indicating that each patient was given 1.3 DDDs of antimicrobials on an average day in SARI ICUs. The median total antimicrobial AD is quite similar to that found in 38 Swedish ICUs (median total antimicrobial AD in local hospitals ICUs 1,072, 1,170 in county hospital ICUs and 1,541 in regional hospital ICUs). Other similar findings from project SARI and the Swedish study were the significant correlation in larger hospitals between total antimicrobial AD and length of stay and the correlation of hospital size and carbapenem use (the larger the hospital the greater the quantity of carbapenems used) [14]. The mean length of stay in SARI ICUs was also correlated with the device-associated nosocomial infection rate, which is likely to influence total antimicrobial use.
So far, there is no explanation for the significant increase in the AD of penicillins with extended spectrum and the significant decrease in the AD of 2nd generation cephalosporins in all types of ICUs. It may be due to the fact that the period of time of antimicrobial use data differs from ICU to ICU (13–29 months). This also applies to the significant decrease in the use of glycopeptides, carbapenems, penicillins with beta-lactamase inhibitor, quinolones and macrolides in interdisciplinary ICUs. If this is the case, this effect can be expected to diminish over time. Mean length of stay not only correlated with total antimicrobial AD, but also with broad spectrum antimicrobials, i.e. quinolones, carbapenems and glycopeptides. Possible explanations for these correlations are the escalation of prolonged antimicrobial therapies and the increased risk of ICU-acquired infections, which are likely to be due to more resistant pathogens.
In the multivariate analysis, length of stay was an independent risk factor for the total AD higher than 75% percentile, as well as a significant correlation in the univariate analysis. Interestingly, a greater number of ICU beds does not increase, but decreases the chance of lying above the 75% percentile.
A high use of glycopeptides was only correlated with the mean CVC utilisation rate. The use of glycopeptides could contribute to the fact that high utilisation rates are likely to result in high ICU-acquired CVC-associated blood stream infection rates, which in turn are likely to result in a high empirical use of glycopeptides to treat these infections (often due to coagulase-negative staphylococci, which are generally resistant to methicillin). It might also contribute to the prophylactic use of glycopeptides for the insertion of CVC, although not recommended in CDC guidelines for the prevention of device-associated blood stream infection [15]. The question as to whether the high use of glycopeptides is related to prophylactic administration or to the treatment of catheter colonisation and/or infection will be the subject of an evaluation of antimicrobial management in SARI ICUs. Other positive correlations found in the univariate analysis, i.e. the incidence density of nosocomial infection and central line-associated blood stream infection rate, could not be confirmed in the multivariate analysis. The number of significant variables of AD for quinolones was also reduced by multiple logistic regression to a single variable, i.e. the rate of CVC. The utilisation rate of CVC may at least serve as an indirect parameter for the severity of illness. Therefore, quinolones and glycopeptides seem to be the preferred antimicrobial agents in more severely ill patients.
Hospital size was not a parameter for high total antimicrobial use density in either the univariate or the multivariate analysis, although several multicentre studies have shown a relationship between increased hospital size and the rate of nosocomial infection (with a subsequent antimicrobial therapy) [16, 17, 18]. However, the Swiss Noso Network recently published data demonstrating that small hospitals do not per se have a lower risk of nosocomial infection [19].
Compared with Swedish ICUs, where the MRSA rate is below 1% and glycopeptide AD ranges between a median of 11 (with infectious disease specialist) and 26 (without), glycopeptide use in SARI ICUs (all ICUs combined) was at least two-fold higher (AD 42). A comparison with US ICARE/AUR data made the transfer from defined daily doses according to WHO to DDDs used in Project ICARE/AUR necessary [13], as well as transfer to the antimicrobial groups applied by ICARE/AUR, which differ from the WHO ATC classification.
In general, German SARI ICUs had higher carbapenem and 2nd generation cephalosporin ADs, whereas physicians in US American ICARE/AUR ICUs administered 3rd generation cephalosporins, ampicillin group antimicrobials and vancomycin much more frequently than their German colleagues [11]. This applied to all types of ICU, namely medical, surgical and interdisciplinary. Administration rates for anti-pseudomonas penicillins depended on the type of ICU involved. MRSA rates in US ICUs exceeding 50% of all Staphylococcus aureus isolates might be the reason for the high use of glycopeptides.
The study has some limitations. Firstly, unit-based data on antimicrobial exposure simply reflect the ecological situation in the individual ICU. Although patient-based data allow further interesting analyses, unit-based data are a very valuable time and cost-effective instrument for the surveillance of antimicrobial use. Secondly, defined daily doses (DDD) and antimicrobial groups were calculated according to definitions given by the WHO (www.whocc.no). Special categories of patients may have an atypical antimicrobial dosing pattern and assessment of the WHO DDDs may not be the best method to characterise antimicrobial use in such specialised patient populations. Thirdly, a comparison of antimicrobial use in different countries and health care systems is prone to bias. There is indirect evidence that patients hospitalised in the USA are more seriously ill, leading to more intense broad spectrum antimicrobial therapy than in Germany, where 7 acute care hospital beds are available per 1,000 inhabitants, compared to 3 per 1000 inhabitants in the USA [20]. Several studies have shown that hospital-wide density of antimicrobial use is much higher in the USA than in Germany or neighbouring European countries [20].
Keeping in mind the limitations of the study, the SARI data can be compared to other local, national and international data on antimicrobial use density in intensive care settings in order to share experiences and improve prudence in the use of antimicrobial agents. The implementation of a surveillance system for ICUs to allow a standardised analysis of antimicrobial use is a basic requirement for auditing the patterns of drug utilisation, the identification of possible problems, for educational or other interventions and for monitoring their outcome [13, 21]. Surveillance data on antimicrobial use allow intra- and inter-ICU comparisons of participating hospitals and form the basis for effective antimicrobial control measures. Furthermore, linking data from SARI with KISS provides the opportunity to analyse additional factors like nosocomial infection rate or device utilisation rates and their influence on antimicrobial use.
Examples of ICUs which establish a monitoring and benchmarking system have been demonstrated by the US American ICARE/AUR project (intensive care antimicrobial resistance epidemiology), which has now been succeeded by the AUR (antimicrobial use and resistance) component of the NNIS (national nosocomial infection surveillance) system [11].
In conclusion, the SARI project presents a first analysis of the data on antimicrobial use in 35 German intensive care units, which will serve as a basis for quality assurance programmes and benchmarking.
References
Anonymous (2001) Proposal for a council recommendation on the prudent use of antimicrobial agents in human medicine. Official Journal of the European Communities L 34/13, 13502–13501
Gaynes R (1997) The impact of antimicrobial use on the emergence of antimicrobial-resistant bacteria in hospitals. Infect Dis Clin North Am 11:757–765
Paladino JA (2000) Economic justification of antimicrobial management programs: implications of antimicrobial resistance. Am J Health Syst Pharm 57 (Suppl 2):S10–S12
Kollef MH, Fraser VJ (2001) Antibiotic resistance in the intensive care unit. Ann Intern Med 134:298–314
Weinstein RA (2001) Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerg Infect Dis 7:188–192
Geffers C, Koch J, Sohr D, Nassauer A, Daschner F, Rueden H, Gastmeier P (2000) [Establishment of a national database for ICU-associated infections. First results from the “Krankenhaus-Infections-Surveillance-System” (KISS)]. Anaesthesist 49:732–737
Meyer E, Jonas D, Schwab F, Rueden H, Gastmeier P, Daschner FD (2003) Design of a surveillance system of antibiotic use and bacterial resistance in German intensive care units (SARI). Infection 31:208–215
ATC index with DDDs (1999) WHO Collaborating Centre for Drug Statistics Methodology, Oslo
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections. Am J Infect Control 16:128–140
National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 to June 2002 (2002) Am J Infect Control 30:458–475
National nosocomial infections surveillance (NNIS) system report. Data summary from January 1992 to June 2001 (2001) Am J Infect Control 29:404–421
Gastmeier P, Schwab F, Geffers C, Rueden H (2002) Is the strict isolation of MRSA patients really necessary? Analysis of data from the German nosocomial infection surveillance system KISS. Fifth International Conference of the Hospital Infection Society, Edingbugh, abstact P5.03
Fridkin SK, Steward CD, Edwards JR, Pryor ER, McGowan JE Jr, Archibald LK, Gaynes RP, Tenover FC (1999) Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project intensive care antimicrobial resistance epidemiology (ICARE) hospitals. Clin Infect Dis 29:245–252
Walther SM, Erlandsson M, Burman LG, Cars O, Gill H, Hoffman M, Isaksso B, Kahlmeter G, Lindgren S, Nilsson L, Olsson-Liljequist B, Hanberger H (2002) Antibiotic prescription practices, consumption and bacterial resistance in a cross section of Swedish intensive care units. Acta Anaesthesiol Scand 46:1075–1081
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick, RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA (2002) Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR 51:1–29
Mertens R, Kegels G, Stroobant A, Reybrouck G, Lamotte JM, Potvliege C, Van Casteren V, Lauwers S, Verschraegen G, Wauters G (1987) The national prevalence survey of nosocomial infections in Belgium, 1984. J Hosp Infect 9:219–229
Ruden H, Gastmeier P, Daschner FD, Schumacher M (1997) Nosocomial and community-acquired infections in Germany. Summary of the results of the First National Prevalence Study (NIDEP). Infection 25:199–202
Vaque J, Rossello J, Arribas JL (1999) Prevalence of nosocomial infections in Spain: EPINE study 1990–1997, EPINE Working Group. J Hosp Infect 43 (Suppl):S105–S111
Sax H, Pittet D (2002) Interhospital differences in nosocomial infection rates: importance of case-mix adjustment. Arch Intern Med 162:2437–2442
OECD Health Data 2000 (2000) OECD, Paris
Emmerson M (2000) Antibiotic usage and prescribing policies in the intensive care unit. Intensive Care Med 26 (Suppl 1):S26–S30
Acknowledgement
Surveillance of antimicrobial use and antimicrobial resistance in intensive care units (SARI) is part of the research network SIR (spread of nosocomial infections and resistant pathogens) supported by the German Ministry of Science and Education. This study was supported by a grant from the German Bundesministerium für Bildung und Forschung (01 KI 9907).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Meyer, E., Schwab, F., Jonas, D. et al. Surveillance of antimicrobial use and antimicrobial resistance in intensive care units (SARI): 1. Antimicrobial use in German intensive care units. Intensive Care Med 30, 1089–1096 (2004). https://doi.org/10.1007/s00134-004-2266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2266-9