Abstract
Objective
To examine whether postoperative mechanical ventilation with lower tidal volumes (VT) has protective effects on inflammatory responses induced by cardiopulmonary bypass (CPB) surgery in smokers and nonsmokers.
Design and setting
Prospective, randomized, controlled clinical trial in the intensive care unit of a university hospital.
Patients and participants
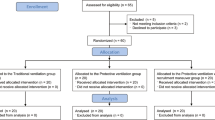
We examined 44 patients (22 smokers, 22 nonsmokers) immediately after uncomplicated CPB surgery.
Interventions
Ventilation was applied for 6 h with either VT of either 6 or 12 ml/kg ideal body weight.
Measurements and results
The time course of serum tumor necrosis factor (TNF) α, interleukin (IL) 6, and IL-8 determined 0, 2, 4, and 6 h after randomization did not differ significantly between the ventilatory strategies. By contrast, in bronchoalveolar lavage fluids sampled after 6 h only TNF-α levels were significantly higher in the high VT group than the low VT group (50±111 pg/ml vs. 1±7 pg/ml). IL-6 and IL-8 concentrations did not differ between groups. Subgroup analysis of patients with serum TNF-α level higher than 0 pg/ml after surgery revealed lower TNF-α serum levels during lower VT ventilation. All observed effects were small, independent of patients’ history of smoking, and were not correlated with duration of ventilation and ICU stay.
Conclusions
Ventilation with lower VT had no or only minor effect on systemic and pulmonary inflammatory responses in patients with healthy lungs after uncomplicated CPB surgery. Our data do not suggest a clinical benefit of using low VT ventilation in these selected patients.
Similar content being viewed by others
Introduction
Despite improvements in perioperative management patients after cardiopulmonary bypass (CPB) surgery frequently require mechanical ventilation. In patients with existing pulmonary and systemic inflammation due to acute lung injury mechanical ventilation with tidal volume (VT) of 10–15 ml/kg ideal body weight and low to moderate levels of positive end-expiratory pressure (PEEP) is known to be associated with higher intra-alveolar and systemic levels of inflammatory mediators [1, 2]. In contrast, mechanical ventilation with moderate to high levels of PEEP and lower VT assure adequate gas exchange is associated with lower intra-alveolar and/or systemic mediator levels [1, 2, 3] and better outcome than in high VT ventilation groups [4, 5].
Cardiac surgery is known to induce a variable degree of systemic inflammatory response syndrome (SIRS), which is severe in 10–35% of cases, and may affect morbidity and mortality [6, 7]. Little, however, is currently known about the effects of mechanical ventilation strategies on postoperative inflammation. In contrast to patients with acute lung injury, recent studies in adult patients with healthy lungs suggest no effect of ventilatory strategies on pulmonary [8] or systemic [8, 9] inflammation before or during surgery. In addition, low VT ventilation is frequently associated with hypercapnia [3, 10], which can increase right ventricular afterload with increased stroke work [11], and may aggravate postoperative metabolic acidosis in cardiac surgical patients, suggesting even preference of higher VT ventilation in these patients.
To test the hypothesis that low VT ventilation has protective effects on inflammatory responses after cardiac surgery with CPB we studied pulmonary and systemic mediator levels using one out of two postoperative ventilator settings in patients recovering from CPB surgery. As tobacco smoking may affect pulmonary immune responses, we prospectively studied the effect of smoking history. For reasons of patients’ safety we included only patients with uncomplicated CPB surgery. Results of this study have been presented at international conferences [12, 13].
Materials and methods
The study enrolled 44 adult patients (22 smokers and 22 nonsmokers) who had undergone elective, uncomplicated cardiac surgery with CPB under general anesthesia. Patients undergoing immunosuppression by drugs or patient’s preoperative underlying disease and those with an elevated white blood cell count (>10×103/µl) or clinical signs of a systemic or pulmonary infection such as fever (>38.5°C), purulent sputum, and pulmonary infiltrates on chest radiography prior to surgery were not included in the study. Patients who developed severe myocardial failure requiring administration of more than 10 µg/min epinephrine or norepinephrine (or any other positive inotrope drug) or intra-aortic balloon pumping after surgery were not included. Patients requiring more than 4 U packed blood cells, fresh-frozen plasma transfusions, or surgical interventions before or during the study period were excluded. Smokers were defined as patients who had smoked during the last 3 months before surgery. The two groups of patients did not differ significantly in demographic or clinical data, including surgery type, bypass time, infusion volumes, and SIRS variables (Table 1).
During CPB surgery patients had received standardized anesthesia using 0.05–0.1 mg/kg midazolam, up to 0.5 MAC isoflurane, continuous infusion of 0.5–1 µg/kg sufentanil per hour, and 0.1–0.2 mg/kg pancuronium bromide. During CPB lungs were not ventilated and rested at continuous positive airway pressure of 5 cmH2O while they were mechanically ventilated with VT of 8–10 ml/kg body weight and a PEEP of 5 cmH2O after CPB. After intensive care unit (ICU) admission patients were ventilated with a standard ventilator (Servo 300, Siemens, Erlangen, Germany). Postoperative analgesia and sedation was maintained with sufentanil (0.1–0.2 µg/kg per hour) and propofol (1–2 mg/kg per hour) for at least 6 h and further as required. Routine postoperative monitoring included invasive measurement of blood pressure, pulse oximetry, and electrocardiography (AS/3, Datex-Ohmeda, Helsinki, Finland). All patients received infusions of crystalloid fluids, with or without additional epinephrine and/or norepinephrine as required to assure a mean arterial pressure above 70 mmHg. Approval of the Bonn University Ethics committee for the study protocol was obtained and all patients gave written informed consent prior to inclusion in the study.
Ventilatory measurements
Gas flow and airway pressure (Paw) were measured by pneumotachography and differential pressure transduction using the same equipment as described previously [9]. Values for VT and minute ventilation (VE) were derived from the integrated gas flow signal.
Physiological gas analysis
Arterial blood gas and pH vales were determined immediately after sampling with standard blood gas electrodes (ABL; Radiometer, Copenhagen, Denmark). Oxygen saturation and hemoglobin in each sample were analyzed using spectrophotometry (OSM3; Radiometer).
Cytokine and chemokine measurements
Bronchoalveolar lavage (BAL) was performed using a bronchoscope with an aliquot of 20 ml sterile isotonic saline in segments of the right lower lobe. If postoperative chest radiography suggested dystelectasis of this lobe, BAL was performed in an unaffected lobe. Commercially available enzyme-linked immunoabsorbent assays were used to measure BAL and plasma levels of interleukin (IL) 6, tumor necrosis factor (TNF) α (Biosource, Ratingen, Germany; detection limit <8 pg/ml), and IL-8 (R&D Systems, Minneapolis, Minn., USA; detection limit 3.5 pg/ml). In addition, IL-2, IL-4, IL-10, interferon γ, and granulocyte-macrophage colony-stimulating factor were measured in BAL and plasma using a Bio-Plex cytokine assay from Bio-Rad (Munich, Germany; detection limit <10 pg/ml). All enzyme-linked immunosorbent assays were performed according to the manufacturers’ guidelines.
Protocol
All patients remained supine throughout the study period. Immediately after ICU admission baseline blood samples for inflammatory mediator measurement were taken, and smokers and nonsmokers were randomly assigned to receive mechanical ventilation either with VT of 12 ml/kg ideal body weight (calculated as previously described [4]; high VT group) or with VT of 6 ml/kg ideal body weight (low VT group) with an inspiratory fraction of O2 (FIO2) and PEEP adjusted according to the algorithm used by the ARDSnet [4]. Ventilator rate was adjusted to maintain PaCO2 between 35 and 50 mmHg and ph higher than 7.25. Blood gases were first considered for analysis 30 min after randomization. Ventilatory measurements were performed after the patients had reached normothermia and stable ventilatory settings (usually after 4 h). Additional blood samples were drawn 2, 4, and 6 h after randomization. Thereafter BAL was performed and data collection was concluded. Hours of mechanical ventilation and length of ICU stay were analyzed in a post hoc fashion.
Statistical analyses
The required sample size was calculated from preliminary data of a previous study on ventilatory strategies in patients during major surgery [8]. To detect differences in the time course of plasma TNF and IL-6 between the ventilatory settings with respect to the subgroups smoker/nonsmoker with the given two-taile parallel design at a significance level of 5% (α=0.05) with a probability of 80% (β=0.20) based on an estimated difference of 0.76 of the parameter’s mean standard deviation the number of patients to be studied in each group is at least 11.
Results are expressed as mean ±standard deviation. All statistical analyses were performed using a statistical software package (Statistica for Windows 5.1, StatSoft, Tulsa, Okla., USA). Data were analyzed using one-way or repeated-measures analysis of variance. If data were not normally distributed (Sharpiro-Wilks’ W test), analysis of variance was performed after log10 transformation to permit the use of parametric statistics. When a significant F ratio was obtained, differences between the means were isolated with the post hoc Tukey’s multiple comparison test. Because distribution of BAL mediator data still differed significantly from normal even after log10 transformation, these data were analyzed by the nonparametric Mann-Whitney U test. Differences were considered to be statistically significant at the level of p<0.05.
Results
All results in our patients were found to be independent of smoking history. Therefore smokers and nonsmokers were analyzed together. Ventilatory variables are shown in Table 2. During mechanical ventilation with low VT, higher ventilator rates (p<0.001) were required to achieve the desired PaCO2 and pH than with high VT mechanical ventilation. Values for VE and the ratio of inspiratory time to expiratory time did not differ between the groups. Adjustment of PEEP according to the ARDS-net [4] algorithm resulted in higher PEEP values in the low VT group (p<0.05), whereas end-inspiratory and maximum airway pressure were higher with high VT (p<0.01 and p<0.001, respectively). Mean airway pressure differed not between the high and low VT group.
Arterial blood gas values are presented in Table 3. The PaO2/FIO2 ratio did not differ significantly between the high and low VT groups. Higher lactic acid levels at admission and lower arterial base excess as well as bicarbonate levels during the study period (p<0.05, p<0.01, and p<0.05, respectively) in the low VT group were associated with lower pH values (p<0.001, Table 3). Acidosis in the low VT group was aggravated by higher PaCO2 (p<0.001, Table 3) despite higher ventilatory rate (p<0.001) and comparable minute ventilation (Table 2). Central venous oxygen saturation determined after 4 h only was not significantly different (low VT 77.1±7.3%. high VT 73.0±6.5%, p=0.066).
The time course of systemic inflammatory mediators was not dependent on postoperative mechanical ventilation strategy (Fig. 1A–C). After 6 h of mechanical ventilation TNF-α levels in BAL fluid were higher in the high VT group than in the low VT group (50±111 vs. 1±7 pg/ml, p<0.01; significant difference persisted when a maximum value was discarded); IL-6 tended to be higher with high VT (987±1942 vs. 128±306 pg/ml, p=0.078) and IL-8, IL-2, IL-4, IL-10, interferon γ, and granulocyte-macrophage colony-stimulating factor displayed no differences between the two ventilation strategies (Fig. 2A–H). Serum levels of IL-2, IL-4, IL-10, interferon γ, and granulocyte-macrophage colony-stimulating factor did not differ between VT high and VT low groups (data not shown). A subgroup of patients (n=18) had serum TNF-α levels above 0 pg/ml after surgery, and reanalysis of these patients revealed lower TNF-α serum levels during lower VT mechanical ventilation (p<0.01; Fig. 3).
Concentrations of TNF-α (a), IL-6 (b), IL-8 (c), IL-2 (d), IL-4 (e), IL-10 (f), interferon (IFN) γ (g), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (h) in bronchoalveolar lavage fluid of all patients after 6 h of mechanical ventilation following cardiac surgery with cardiopulmonary bypass. Note that the scaling differs between plots. **p=0.01 VT high vs. VT low
Post hoc analysis of plasma TNF-α course (mean ±SD) during the first 6 h of mechanical ventilation after ICU admission following cardiac surgery with cardiopulmonary bypass in a subgroup of 18 patients with plasma TNF-α greater than 0 pg/ml at time of ICU admission. Overall differences between groups were significant at p<0.01; *p<0.05 vs. VT low, **p<0.01 vs. VT low
No difference in epinephrine/norepinephrine requirement was observed between the groups (data not shown), and postoperative chest radiography showed no infiltrates or pulmonary edema. Time on postoperative mechanical ventilation did not differ between groups (Table 1; p=0.21), but duration of ICU treatment tended to be lower in the high VT group (Table 1; p=0.055). No significant correlation between BAL or serum mediator levels after 6 h and ventilation time or ICU length of stay was observed except for serum IL-8 and time on ventilation (r=0.32, p<0.05). In a subgroup of five patients with pulmonary TNF levels higher than 100 pg/ml time on mechanical ventilation (13.4±4.3 h) and duration of ICU treatment (1.4±0.9 days) was not significantly higher than the means of either group.
Discussion
Cardiac surgery with CPB imposes considerable trauma to the lungs, as indicated by significantly impaired oxygenation [14, 15], and might be associated with pulmonary and systemic inflammation [16, 17, 18, 19]. Activation of the humoral and cellular immune system with enhanced release of cytokines can lead to increased capillary permeability, respiratory distress, hypo- or hyperdynamic circulatory dysregulation, and subsequent multiple organ dysfunction [6, 7]. Effects of mechanical ventilation strategies on pulmonary inflammatory responses during recovery from cardiac surgery have not been studied previously.
Our study protocol has advantages and disadvantages. The main advantages are that the study design assured minimization of patients’ individual risk for being compromised by ventilatory strategies or diagnostic procedures. For reasons of patient safety we did not include patients with independent predictors for development of acute respiratory distress syndrome (ARDS; incidence <0.5% [20]) such as shock and multiple transfusions. In additions, considering that ARDS has been found not to occur before the second postoperative day in epidemiological studies [20], the likelihood of one of our selected patients developing ARDS within 6 h was by design very unlikely and was indeed not observed in any patient during the clinical course. According to this and because to our knowledge there is no evidence that ventilation with a VT of 12 ml/kg ideal body weight causes harm in adult patients with normal lungs [8, 9, 21, 22], it was not explicitly stated in the patient information and consent forms approved by our ethical review board that ventilation with a VT of 12 ml/kg is associated with poorer outcome variables in patients with acute lung injury or ARDS [4]. Although all our patients were informed about risk factors which may be associated with either ventilatory strategy before they agreed to participate, whether to mention this in our consent form may be matter of debate. Recently published recommendations for informed consent forms in critical care clinical trials should help to improve design of future studies with respect to this issue [2]. Furthermore, a VT of 12 ml/kg might not be considered as “standard of care.” Ethical issues of using “standard of care” vs. protocol groups in low and high VT ventilation studies have been discussed elsewhere [23, 24].
Since ventilation after CPB is often interrupted, for example by the need for surgical interventions, hemodynamic instability during weaning from CPB which might limit the use of PEEP, and manual ventilation during transportation to the ICU, we decided to randomize patients immediately after ICU arrival when conditions were controlled. However, we cannot exclude that ventilation with intermediate VT of 8–10 ml/kg for less than 2 h after CPB and before ICU admission (Table 1) might have affected our results.
Although BAL is considered a safe procedure even in patients with ARDS or acute lung injury [25, 26], BAL itself has been shown to effect a significant increase in plasma cytokines levels [27]. We therefore performed only one mini-BAL (20 ml lavage volume), thereby accepting methodological limitations [28]. Furthermore, we studied only patients with uncomplicated cardiac surgery and without severe inflammatory response syndrome, which limits our conclusion to these kinds of patients.
Our study found only minor effects of mechanical ventilation on pulmonary inflammation. Although we observed significantly higher levels of TNF-α and a tendency towards higher IL-6 levels in the BAL fluid of patients ventilated with high VT, these statistical difference was caused by only a few patients and were not correlated with clinical outcome. The nonuniform distribution of these data suggests individual differences in the inflammatory responses to cardiac surgery with CPB even in patients with relatively low levels of inflammatory markers. To address this we performed a post hoc analysis of patients with elevated TNF-α plasma levels at intensive care unit admission. Elevated systemic TNF-α levels decreased more rapidly in patients ventilated with low VT and higher PEEP. However, because these differences were small and refer to one single cytokine as part of a complex interaction of inflammatory mediators, the findings are unlikely to bear clinical relevance. This is supported by clinical trials in patients with sepsis syndrome which have observed no beneficial effects of anti-TNF-α treatment [29]. Individual differences in TNF-α response to cardiac surgery may also be influenced by allele frequency and genotype distribution of a biallelic TNF-α gene polymorphism [30]. Because this study was not designed and powered to investigate an effect of genetic polymorphisms on inflammatory responses after cardiac surgery [31], we cannot exclude an influence of these factors.
In patients with acute lung injury or acute respiratory distress syndrome mechanical ventilation with low VT ventilation of 6 ml/kg ideal body weight with moderate [4, 32] or high levels of PEEP [5] has been observed to decrease mortality in acute lung injury or the acute respiratory distress syndrome when compared to mechanical ventilation with high VT of at least 12 ml/kg ideal body weight. The ARDSnet trial and other studies found that low VT ventilation was associated with lower pulmonary and/or systemic inflammatory mediator concentrations [1, 2, 3, 4]. In contrast, 1 h of ventilation in patients with normal lungs and without surgery does not alter plasma levels of inflammatory mediators [9]. The present work extends these studies to patients without lung injury who underwent severe surgery. Although cardiac surgery with CPB-like acute lung injury is characterized by inflammation, we did not observe significant differences in systemic inflammatory markers depending on the mechanical ventilation strategy during the first 6 h after surgery. This is in line with our recently reported findings showing no effect of mechanical ventilator settings on mild to moderate inflammatory responses during major thoracic or abdominal surgery [8] and those of a recent study by Koner and coworkers [21] who also observed no differences in plasma cytokine levels at different ventilatory settings during and 2 h after CPB surgery. Unfortunately, the latter study did not investigate pulmonary inflammatory responses [21].
In our patients systemic mediator levels were only moderately elevated, and no patient had plasma IL-6 levels higher than 1000 pg/ml, a threshold previously used to identify patients with severe SIRS following cardiac surgery [7]. Clinical studies reported incidences of a severe SIRS after cardiac surgery with CPB in 4–44% of patients [34]. However, occurrence of severe SIRS is markedly increased in high-risk patients with low ejection fraction [7, 34], who were not included in our study. Other risk factors, including long CPB time, advanced age, infused fluid and blood volumes did not differ between groups in this study.
The somewhat conflicting observations in patients with and without previous pulmonary inflammation and the observed differences between our patients with elevated systemic TNF-α levels may be explained by a two-hit model. According to this model, pulmonary inflammation must already be present (first hit) for injurious mechanical ventilation (second hit) to aggravate the inflammatory response. This hypothesis is supported by several experimental studies showing elevated inflammatory responses to high VT mechanical ventilation following an inflammatory first hit [35, 36, 37]. In our patients cardiac surgery with CPB does not appear to be a sufficiently strong first hit in terms of lung injury to result in clinically significant differences between mechanical ventilation settings as the second hit. History of smoking did not prove an additional hit in this context. These conclusions cannot necessarily be extended to patients with severe SIRS after cardiac surgery, who were not included here.
The two mechanical ventilation strategies resulted in comparable arterial oxygenation, which was mild to moderately impaired after cardiac surgery. Despite the higher ventilatory rate PaCO2 was higher in the low VT group and acidosis was aggravated in the low VT group. Hypercapnia in the low VT ventilation group has been suggested to have lung protective effects by itself and may have contributed to the observed differences in inflammatory responses [38]. Although we did not measure cardiac output in this study, lack of differences in central venous oxygen saturation after adequate rewarming time of 4 h does not suggest major differences in systemic blood flow between groups. Thus differences in metabolic acidosis might be explained by postoperative patient status. However, the lack of a significant correlation between acidosis and length of stay in the ICU (data not shown) does not support the idea that more severe acidosis in the low VT group contributed to the trend towards longer stay in the intensive care unit of these patients.
In conclusion, mechanical ventilation with lower VT for 6 h in patients with mild to moderate inflammatory responses after cardiac surgery resulted in no or only minor differences in pulmonary and systemic mediator concentrations, independently of the patients’ smoking history. The finding that patients with elevated TNF-α levels after surgery showed slightly lower TNF-α plasma levels during lower VT ventilation provides further support for the two-hit theory. Based solely on our studies in patients with uncomplicated cardiac surgery, we cannot recommend preferring either ventilatory strategy studied here.
References
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282:54–61
Silverman HJ, Luce JM, Lanken PN, Morris AH, Harabin AL, Oldmixon CF, Thompson BT, Bernard GR (2005) Recommendations for informed consent forms for critical care clinical trials. Crit Care Med 33:867–882
Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP (2005) Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33:1–6
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi FG, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T, Haverich A, Schlag G, Borst HG (1996) Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 61:1714–1720
Kilger E, Weis F, Briegel J, Frey L, Goetz AE, Reuter D, Nagy A, Schuetz A, Lamm P, Knoll A, Peter K (2003) Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit Care Med 31:1068–1074
Wrigge H, Uhlig U, Zinserling J, Behrends-Callsen E, Ottersbach G, Fischer M, Uhlig S, Putensen C (2004) The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg 98:775–81, table
Wrigge H, Zinserling J, Stüber F, Spiegel T, Hering R, Wetegrove S, Hoeft A, Putensen C (2000) Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 93:1413–1417
Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR, Slutsky AS (1998) Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 338:355–361
Viitanen A, Salmenpera M, Heinonen J (1990) Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 73:393–400
Wrigge H, Uhlig U, Menzenbach J, Zinserling J, Uhlig S, Putensen, C (2003) Does postperative ventilatory strategy affect inflammatory response to cardiac surgery in smokers and non-smokers (abstract)? Am J Respir Crit Care Med 167[Suppl]:A905
Wrigge H, Uhlig U, Menzenbach J, Zinserling J, Uhlig S, Putensen, C (2003) Inflammatory effects of conventional and lower tidal volume ventilation after cardiac surgery (abstract). Intensive Care Med 29 [Suppl 1]:S307
Hachenberg T, Tenling A, Nystrom SO, Tyden H, Hedenstierna G (1994) Ventilation-perfusion inequality in patients undergoing cardiac surgery. Anesthesiology 80:509–519
Hachenberg T, Tenling A, Hansson HE, Tyden H, Hedenstierna G (1997) The ventilation-perfusion relation and gas exchange in mitral valve disease and coronary artery disease. Implications for anesthesia, extracorporeal circulation, and cardiac surgery. Anesthesiology 86:809–817
Boldt J, Osmer C, Linke LC, Dapper F, Hempelmann G (1995) Circulating adhesion molecules in pediatric cardiac surgery. Anesth Analg 81:1129–1135
Nathan N, Denizot Y, Cornu E, Jauberteau MO, Chauvreau C, Feiss P (1997) Cytokine and lipid mediator blood concentrations after coronary artery surgery. Anesth Analg 85:1240–1246
Berendes E, Mollhoff T, Van Aken H, Schmidt C, Erren M, Deng MC, Weyand M, Loick HM (1997) Effects of dopexamine on creatinine clearance, systemic inflammation, and splanchnic oxygenation in patients undergoing coronary artery bypass grafting. Anesth Analg 84:950–957
Gilliland HE, Armstrong MA, McMurray TJ (1999) The inflammatory response to pediatric cardiac surgery: correlation of granulocyte adhesion molecule expression with postoperative oxygenation. Anesth Analg 89:1188–1191
Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F (2001) Incidence and predictors of ARDS after cardiac surgery. Chest 119:884–888
Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N (2004) Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med 30:620–626
International Consensus Conferences in Intensive Care Medicine (1999) Ventilator-associated lung injury in ARDS. American Thoracic Society, European Society of Intensive Care Medicine, Societe de Reanimation Langue Francaise. Intensive Care Med 25:1444–1452
Miller FG, Silverman HJ (2004) The ethical relevance of the standard of care in the design of clinical trials. Am J Respir Crit Care Med 169:562–564
Brower RG, Bernard G, Morris A (2004) Ethics and standard of care in clinical trials. Am J Respir Crit Care Med 170:198–199
Montravers P, Gauzit R, Dombret MC, Blanchet F, Desmonts JM (1993) Cardiopulmonary effects of bronchoalveolar lavage in critically ill patients. Chest 104:1541–1547
Steinberg KP, Mitchell DR, Maunder RJ, Milberg JA, Whitcomb ME, Hudson LD (1993) Safety of bronchoalveolar lavage in patients with adult respiratory distress syndrome. Am Rev Respir Dis 148:556–561
Krause A, Hohberg B, Heine F, John M, Burmester GR, Witt C (1997) Cytokines derived from alveolar macrophages induce fever after bronchoscopy and bronchoalveolar lavage. Am J Respir Crit Care Med 155:1793–1797
Pugin J (2002) Is the ventilator responsible for lung and systemic inflammation? Intensive Care Med 28:817–819
Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA 273:934–941
Schroeder S, Borger N, Wrigge H, Welz A, Putensen C, Hoeft A, Stüber F (2003) A tumor necrosis factor gene polymorphism influences the inflammatory response after cardiac operation. Ann Thorac Surg 75:534–537
Tomasdottir H, Hjartarson H, Ricksten A, Wasslavik C, Bengtsson A, Ricksten SE (2003) Tumor necrosis factor gene polymorphism is associated with enhanced systemic inflammatory response and increased cardiopulmonary morbidity after cardiac surgery. Anesth Analg 97:944–949
Hickling KG, Henderson SJ (1990) Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16:372–377
Stüber F, Wrigge H, Schroeder S, Wetegrove S, Zinserling J, Hoeft A, Putensen C (2002) Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med 28:834–841
Johnson MR (1999) Low systemic vascular resistance after cardiopulmonary bypass: are we any closer to understanding the enigma? Crit Care Med 27:1048–1050
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS (1997) Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99:944–952
Chiumello D, Pristine G, Slutsky AS (1999) Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 160:109–116
Held HD, Boettcher S, Hamann L, Uhlig S (2001) Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med 163:711–716
Laffey JG, O’Croinin D, McLoughlin P, Kavanagh BP (2004) Permissive hypercapnia—role in protective lung ventilatory strategies. Intensive Care Med 30:347–356
Acknowledgements
The authors thank Dörte Karp and Renate Bergmann for their excellent technical assistance. This study was performed at the Department of Anesthesiology and Intensive Care Medicine, University of Bonn, Germany. Results of this study have been presented in part during annual meetings of the ATS in 2003, Seattle, Wash., USA, and ESICM in 2003, Amsterdam, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the BONFOR Forschungsförderung (project O-117.0006), University of Bonn, Germany, and from the Deutsche Forschungsgemeinschaft (Pu 219/1-1, and Uh 88/4-1), Bonn, Germany, and by departmental funding.
Rights and permissions
About this article
Cite this article
Wrigge, H., Uhlig, U., Baumgarten, G. et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Med 31, 1379–1387 (2005). https://doi.org/10.1007/s00134-005-2767-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2767-1