Abstract
Objective
The aim was to investigate the effects of cardiopulmonary bypass (CPB) on plasma levels of the vascular growth factors, angiopoietin (angpt)-1, angpt-2, and vascular endothelial growth factor (VEGF).
Design
The design was a prospective, clinical investigation.
Setting
The setting was a 12-bed pediatric cardiac intensive care unit of a tertiary children’s medical center.
Patients
The patients were 48 children (median age, 5 months) undergoing surgical correction or palliation of congenital heart disease who were prospectively enrolled following informed consent.
Interventions
There were no interventions in this study.
Measurements and results
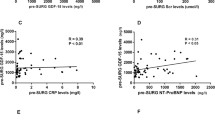
Plasma samples were obtained at baseline and at 0, 6, and 24 h following CPB. Angpt-1, angpt-2, and VEGF levels were measured via commercial ELISA. Angpt-2 levels increased by 6 h (0.95, IQR 0.43–2.08 ng mL−1 vs. 4.62, IQR 1.16–6.93 ng mL−1, P < 0.05) and remained significantly elevated at 24 h after CPB (1.85, IQR 0.70–2.76 ng mL−1; P < 0.05). Angpt-1 levels remained unchanged immediately after CPB, but were significantly decreased at 24 h after CPB (0.64, IQR 0.40–1.62 ng mL−1 vs. 1.99, IQR 1.23–2.63 ng mL−1, P < 0.05). Angpt-2 levels correlated significantly with cardiac intensive care unit (CICU) length of stay (LOS) and were an independent predictor for CICU LOS on subsequent multivariate analysis.
Conclusions
Angpt-2 appears to be an important biomarker of adverse outcome following CPB in children.


Similar content being viewed by others
References
Tarnok A, Schneider P (2001) Pediatric cardiac surgery with cardiopulmonary bypass: pathways contributing to transient systemic immune suppression. Shock 16:24–32
Wan S, LeClerc JL, Vincent JL (1997) Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 112:676–692
Zhang S, Wang S, Li Q, Yao S, Zeng B, Ziegelstein RC, Hu Q (2005) Capillary leak syndrome in children with C4A-deficiency undergoing cardiac surgery with cardiopulmonary bypass: a double-blind, randomised controlled study. Lancet 366:556–562
Wheeler DS, Dent CL, Manning PB, Nelson DP (2008) Factors prolonging length of stay in the cardiac intensive care unit following the arterial switch operation. Cardiol Young 18:41–50
Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ (2003) Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med 31:28–33
van Dongen EI, Glandsorp AG, Mildner RJ, McCrindle BW, Sakopoulos AG, VanArdsell G, Williams WG, Bohn D (2003) The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 126:703–710
Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, Almirall D, Wessel DL, Jonas RA, Wernovsky G (2003) Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr 143:67–73
Seghave MC, Grabitz RG, Duchateau J, Busse S, Dabritz S, Koch D, Alzen G, Hornchen H, Messmer BJ, Von Bernuth G (1996) Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg 112:687–697
Abrahamov D, Erez E, Tamariz M, Dagan O, Pearl E, Abrahamov Y, Gendel B, Desai N, Kats J, Vidne B, Barak V (2002) Plasma vascular endothelial growth factor level is a predictor of the severity of postoperative capillary leak syndrome in neonates undergoing cardiopulmonary bypass. Pediatr Surg Int 18:54–59
Starnes SL, Duncan BW, Kneebone JM, Rosenthal GL, Jones TK, Grifka RG, Cecchin F, Owens DJ, Fearneyhough C, Lupinetti FM (2000) Vascular endothelial growth factor and basic fibroblast growth factor in children with cyanotic congenital heart disease. J Thorac Cardiovasc Surg 119:534–539
Himeno WH, Akagi T, Furui J, Maeno Y, Ishii M, Kosai K, Murohara T, Kato H (2003) Increased angiogenic growth factor in cyanotic congenital heart disease. Pediatr Cardiol 24:127–132
Ootaki Y, Yamaguchi M, Yoshimura N, Oka S, Yoshida M, Hasegawa T (2003) Vascular endothelial growth factor in children with congenital heart disease. Ann Thorac Surg 75:1523–1526
Brindle NPJ, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98:1014–1023
Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C (2005) Protective role of angiopoietin-1 in endotoxic shock. Circulation 111:97–105
Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP (2006) Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3:e46
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239
Chong AY, Caine GJ, Freestone B, Blann AD, Lip GYH (2004) Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor Tie-2 levels in congestive heart failure. J Am Coll Cardiol 43:423–428
Lee KW, Lip GYH, Blann AD (2004) Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor Tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 110:2355–2360
Giuliano JS Jr, Lahni PM, Bigham MT, Nelson DP, Manning PB, Bogenshutz L, VanVliet T, Wong HR, Wheeler DS (2007) Angiopoietin expression in children following cardiopulmonary bypass. Circulation 116:II-515
Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI (2002) Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123:110–118
Wernovsky G, Wypij D, Jonas RA, Mayer JEJ, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bpyass and circulatory arrest. Circulation 92:2226–2235
Schroeder VA, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP (2003) Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation 107:2823–2828
Kozik DJ, Tweddell JS (2006) Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg 81:S2347–S2354
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156
Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K (1997) Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 100:2072–2078
Verrier ED, Morgan EN (1998) Endothelial response to cardiopulmonary bypass surgery. Ann Thorac Surg 66:S17–S19
Lobov IB, Brooks PC, Lang RA (2002) Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99:11205–11210
Kim I, Moon SO, Park SK, Chae SW, Koh GY (2001) Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res 89:477–479
Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A (2005) Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744
Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C (2007) Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med 35:199–206
Giuliano JS Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS (2007) Admission angiopoietin levels in children with septic shock. Shock 28:650–654
Thompson LD, McElhinney DB, Findlay P, Miller-Hance W, Chen MJ, Minami M, Petrossian E, Parry AJ, Reddy VM, Hanley FL (2001) A prospective, randomized study comparing volume-standardized modified and conventional ultrafiltration in pediatric cardiac surgery. J Thorac Cardiovasc Surg 122:220–228
Acknowledgments
We would like thank Tracey VanVliet and Lois Bogenschutz in the Cardiology research department at Cincinnati Children’s Hospital for their assistance with this project. The study was supported by the National Institutes of Health KO8 GM077432 (DSW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giuliano, J.S., Lahni, P.M., Bigham, M.T. et al. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med 34, 1851–1857 (2008). https://doi.org/10.1007/s00134-008-1174-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1174-9