Abstract
Introduction and hypothesis
To determine if abobotulinumtoxin A (AboBTXA) is an effective treatment for interstitial cystitis/bladder pain syndrome (IC/BPS).
Methods
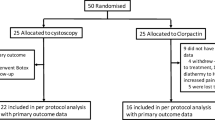
We performed a double-blind study of 54 women with severe, refractory IC from three referral centres whom we randomly allocated to treatment with hydrodistension + injection of normal saline or to hydrodistension + injection with AboBTXA. The O’Leary-Sant questionnaire consists of problem (OLS-PI) and symptom (OLS-PI) index scores, and bladder diary data were compared between AboBTXA and control patients at baseline and at 3 months of follow-up. Measurements were made beyond 3 months, but no further randomised comparison was possible due to the ability of nonresponsive patients in either group to have AboBTXA treatment.
Results
Complete data were available in 50 patients, and in both groups, OLS questionnaires showed improvement at 3 months. Only the OLS-PI was improved in the AboBTXA group (p = 0.04). At 3 months, no difference was found in either OLS-SI or total OLS score. Twelve patients had urinary tract infection (UTI) treated during the follow-up period, which confounded results. In the 38 patients without UTI, there was improvement in total OLS score (p = 0.02), OLS-PI (0.08), and OLS-SI (p = 0.008) for the AboBTXA group at 3 months. Only five AboBTXA compared with two control patients had a 50 % reduction in OLS score.
Conclusions
For chronic refractory IC/BPS patients, AboBTXA was associated with no overall improvement in total OLS score, although significant benefit was noted in a small number of patients. The absence of posttreatment UTI was associated with a better response to AboBTXA.

Similar content being viewed by others
Abbreviations
- OnaBTXA:
-
Onabotulinum A toxin (Botox®)
- AboBTXA:
-
Abobotulinum A toxin (Dysport®)
- UTI:
-
Urinary tract infection
- IC/BPS:
-
Interstitial cystitis bladder pain syndrome
- OLS:
-
O’Leary-Sant questionnaire score
- BCG:
-
Bacille Calmette-Guerin
References
Smith C, Radziszewski P, Borkowski A, Somogyi G, Boone T, Chancellor MB (2004) Botulinum toxin a has antinocicpetive effects in treating interstitial cystitis. Urology 64(5):871–875
Giannantoni A, Porena M, Costantini E, Zucchi A, Mearini L, Mearini E (2008) BotulinumA toxin intravesical injection in patients with painful bladder syndrome: 1-year followup. J Urol 179(3):1031–1034
Ramsay AK, Small DR, Conn IG (2007) IntravesicalBotulinum toxin type A in chronic interstitial cystitis: results of a pilot study. Surg J R Coll Surg Edinb Ireland 5(6):331–333
Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB (2004) Intravesical Botulinum Toxin A administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 172(4):1529–1532
Pinto R, Silva A, Lopes T, Silva JF, Silva CM, Cruz FR, Dinis PO (2009) Intra-trigonal injection of Botulinum toxin A in patients with bladder pain syndrome.Results at 9 months of follow-up. J Urol 181(4,supplement):20
El-Bahnasy AE, Faharat YA, El-Bendary M, Taha MR, El-Damhogy M, Mourad S (2005) A randomised controlled trial of Bacillus Calmette-Guerin and Botulinum Toxin A for the treatment of refractory interstitial cystitis. UIJ 12(6):1944
Gillenwater JY, Wein AJ (1987) Summary of the National Institiute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial cystitis, National Institiutes of Health, Bethesda, Maryland, August 28-29. J Urol 1987(140):203–206
Karsai S, Raulin C (2010) Botox and Dysport: Is there a dose conversion ratio in dermatology and aesthetic medicine? J Am Acad Dermatol 62(2):346–347
Manstein C, Beidas OE (2011) Comparing Clinical Efficacy of Botox and Dysport in a Small Group of Patients. Plastic Reconstr Surg 128(1):26e
Jacobson NS, Truax P (1991) Clincial Significance: A Statistical Approach to Defining Meaningful Change in Psychotherapy Research, J. Consult Clin Psychol 59(1):12–19
Deyo RA, Diehr P, Patrick DL (1991) Reproducibility and Responsiveness of Health Status Measures. Control Clin Trials 12:142S–158S
Van de Merwe JNJ, Bouchelouche P, Bouchelouche K, Cervigni M, Daha L, Elneil S, Fall M, Hohlbrugger G, Irwin P, Mortensen S, van Ophoven A, Osborne J, Peeker R, Richter B, Riedl C, Sairanen J, Tinzl M, Wyndaele J-J (2008) Diagnostic Criteria, Classification, and Nomenclature for Painful Bladder Syndrome/Interstitial Cystitis: An ESSIC Proposal. Eur Urol 53:60–67
Kuo HC (2010) questionnaire) Preliminary results of suburothelial injection of botulinum a toxin in the treatment of chronic interstitial cystitis. Urol Int 75:170–174
Lee CL, Kuo HC (2013) Intravesical botulinum toxin a injections do not benefit patients with ulcer type interstitial cystitis. Pain Phys 16(2):109–116
Davies A, Chahal R, Inman R, Urwin G (2006) Intravesicalbotulinum A toxin (Botox)-- does it have a role in the management of interstitial cystitis. Eur Urol, Suppl 5(suppl 2):22
Sairanen J, Leppilahti M, Tammela TL, Paananen I, Aaltomaa S, Taari K, Ruutu M (2009) Evaluation of health-related quality of life in patients with painful bladder syndrome/interstitial cystitis and the impact of four treatments on it. Scand J Urol Nephrol 43(3):212–219
Flynn MK, Amundsen CL, Perevich MA, Liu F, Webster GD (2009) Outcome of a randomized, double blind, placebo controlled trial of Botulinum A toxin for refractory overactive bladder. J Urol 181(6):2608–2615
Liu HT, Tyagi P, Chancellor MB, Kuo HC (2009) Urinary nerve growth factor level is increased in patients with interstitial cystitis/ bladder pain syndrome and decreased in responders to treatment. BJU Int 104(10):1476–1481
Jeffrey S, Fynes M, Lee F, Williams L, Morley R (2007) Efficacy and complications of intradetrusor injection with botulinum toxin A in patients with refractory idiopathic detrusor overactivity. BJU Int 100(6):1302–1306
Conflict of Interest
PD is on Advisory Board for Allergan
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLS 63 kb)
Rights and permissions
About this article
Cite this article
Manning, J., Dwyer, P., Rosamilia, A. et al. A multicentre, prospective, randomised, double-blind study to measure the treatment effectiveness of abobotulinum A (AboBTXA) among women with refractory interstitial cystitis/bladder pain syndrome. Int Urogynecol J 25, 593–599 (2014). https://doi.org/10.1007/s00192-013-2267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2267-8