Abstract
The effects of ozone \(({\hbox {O}}_{3})\) or nitrogen dioxide \(({\hbox {NO}}_{2})\) as oxygen atom precursors on the characteristic length-scales of low-temperature chemistry (LTC)-affected detonation propagating in dimethyl \({\hbox {ether}}{-}{\hbox {O}}_{2}{-}{\hbox {CO}}_{2}\) mixtures were investigated using the Zeldovich–von Neumann–Döring model. The effect of these two additives on the energy release dynamics and chemical kinetics was analyzed. Under some conditions, up to three steps of energy release were observed. Ozone strengthens the LTC which results in a decrease in the induction zone length along with an increase of the energy release rate. The addition of \({\hbox {NO}}_{2}\) provides chemical pathways which lead to a bypass of the intermediate-temperature chemistry and a decrease in the separation distance between the first and the second steps of energy release. An increase of the energy release rate is also observed for the two first peaks. Among the two additives tested, ozone appears as the most promising to experimentally observe LTC-affected detonation with multi-stage energy release.







Similar content being viewed by others
References
Lee, J.H.S.: The Detonation Phenomenon. Cambridge University Press, Cambridge (2018). https://doi.org/10.1017/CBO9781316226926
Liang, W., Mevel, R., Law, C.K.: Role of low-temperature chemistry in detonation of n-heptane/oxygen mixtures. Combust. Flame 193, 463–470 (2018). https://doi.org/10.1016/j.combustflame.2018.03.035
Presles, H., Desbordes, D., Guirard, M., Guerraud, C.: Gaseous nitromethane and nitromethane-oxygen mixtures: a new detonation structure. Shock Waves 6, 111–114 (1996). https://doi.org/10.1007/BF02515194
Manzhalei, V.I.: Fine structure of the leading front of a gas detonation. Combust. Explos. Shock Waves 13, 402–404 (1977). https://doi.org/10.1007/BF00740325
Liang, W., Law, C.K.: Extended flammability limits of n-heptane/air mixtures with cool flames. Combust. Flame 185, 75–81 (2017). https://doi.org/10.1016/j.combustflame.2017.06.015
Ng, H.D., Ju, Y., Lee, J.H.S.: Assessment of detonation hazards in high-pressure hydrogen storage from chemical sensitivity analysis. Int. J. Hydrogen Energy 32, 93–99 (2007). https://doi.org/10.1016/j.ijhydene.2006.03.012
He, L., Clavin, P.: On the direct initiation of gaseous detonations by an energy source. J. Fluid Mech 277, 227–248 (1994). https://doi.org/10.1017/S0022112094002740
Eckett, C.A., Quirk, J.J., Shepherd, J.E.: The role of unsteadiness in direct initiation of gaseous detonations. J. Fluid Mech 421, 147–183 (2000). https://doi.org/10.1017/S0022112000001555
Schultz, E.: Detonation Diffraction Through an Abrupt Area Expansion. Ph.D. thesis, California Institute of Technology (2000). http://resolver.caltech.edu/CaltechETD:etd-11122003-180459
Lee, J.H.S.: Dynamic parameters of gaseous detonations. Annu. Rev. Fluid Mech. 16, 311–336 (1984). https://doi.org/10.1146/annurev.fl.16.010184.001523
Mevel, R., Melguizo-Gavilanes, J., Radulescu, M.: ZND structure of cool detonation in dimethyl ether–oxygen–carbon dioxide mixtures. Proceedings of the 11th Asia-Pacific Conference on Combustion (2018)
Radulescu, M.I., Borzou, B.: Dynamics of detonations with a constant mean flow divergence. J. Fluid Mech. 845, 346–377 (2018). https://doi.org/10.1017/jfm.2018.244
Bhagatwala, A., Luo, Z., Shen, H., Sutton, J.A., Lu, T., Chen, J.: Numerical and experimental investigation of turbulent DME jet flames. Proc. Combust. Inst 35, 1157–1166 (2015). https://doi.org/10.1016/j.proci.2014.05.147
Pan, L., Kokjohn, S., Huang, Z.: Development and validation of a reduced chemical kinetic model for dimethyl ether combustion. Fuel 160, 165–177 (2015). https://doi.org/10.1016/j.fuel.2015.07.066
Zhao, Z., Chaos, M., Kazarov, A., Dryer, F.L.: Thermal decomposition reaction and a comprehensive kinetic model of dimethyl ether. Int. J. Chem. Kinet. 40, 1–18 (2008). https://doi.org/10.1002/kin.20285
Zhao, H., Yang, X., Ju, Y.: Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry. Combust. Flame 173, 187–194 (2016). https://doi.org/10.1016/j.combustflame.2016.08.008
Mével, R., Javoy, S., Lafosse, F., Chaumeix, N., Dupré, G., Paillard, C.-E.: Hydrogen–nitrous oxide delay time: shock tube experimental study and kinetic modelling. Proc. Combust. Inst. 32, 359–366 (2009). https://doi.org/10.1016/j.proci.2008.06.171
Mével, R., Shepherd, J.E.: Ignition delay-time behind reflected shock waves of small hydrocarbons–nitrous oxide(–oxygen) mixtures. Shock Waves 25, 217–229 (2015). https://doi.org/10.1007/s00193-014-0509-4
Shepherd, J.E.: Chemical kinetics of hydrogen-air-diluent detonations. Prog. Astronaut. Aeronaut. 106, 263–293 (1986). https://doi.org/10.2514/5.9781600865800.0263.0293
Lutz, A.E., Kee, R.J., Miller, J.A.: SENKIN: a fortran program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis. In: Sandia National Labs., Livermore, CA (USA), No. SAND-87-8248 (1988)
Crane, J., Shi, X., Singh, A.V., Tao, Y., Wang, H.: Isolating the effect of induction length on detonation structure: hydrogen-oxygen detonation promoted by ozone. Combust. Flame 200, 44–52 (2019). https://doi.org/10.1016/j.combustflame.2018.11.008
Dai, P., Chen, Z.: Effects of NOx addition on autoignition and detonation development in DME/air under engine-relevant conditions. Proc. Combust. Inst. 37, 4813–4820 (2019). https://doi.org/10.1016/j.proci.2018.06.063
Dorofeev, S., Sidorov, V., Dvoinishnikov, A.: Deflagration to detonation transition in large confined volume of lean hydrogen-air mixtures. Combust. Flame 104, 95–110 (1996). https://doi.org/10.1016/0010-2180(95)00113-1
Acknowledgements
RM was supported by a start-up fund of the Center for Combustion Energy of Tsinghua University, the Thousand Young Talents Program of China, and the 1000 Young Talents Matching Fund of Tsinghua University. YH was funded by China Postdoctoral Science Foundation (Grant Number 2019M650674).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Ciccarelli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is based on work that was presented at the 27th International Colloquium on the Dynamics of Explosions and Reactive Systems, Beijing, China, July 28–August 2, 2019.
Appendices
Appendix 1: Effect of the amount of additive
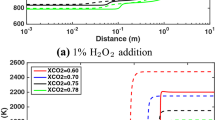
Figure 8 shows the temperature profiles obtained using ZND simulations for \({\hbox {DME}}{-}{\hbox {O}}_{2}{-}{\hbox {CO}}_{2}\) mixtures with various amounts of \({\hbox {O}}_{3}\) and \({\hbox {NO}}_{2}\) addition. The effect of \({\hbox {O}}_{3}\) is more pronounced as \({\hbox {CO}}_{2}\) dilution is increased. At \(X_{{\hbox {CO}}_{2}}>0.70\), an addition of 100 ppm of \({\hbox {O}}_{3}\) can reduce the induction zone length of the first stage of ERR by 5 to 10 times. For \({\hbox {NO}}_{2}\), an addition of 1000 ppm is required to induce a clearly visible impact on the temperature profiles, whatever the \({\hbox {CO}}_{2}\) dilution is.
ZND temperature profiles of \({\hbox {DME}}{-}{\hbox {O}}_{2}{-}{\hbox {CO}}_{2}\) mixtures without and with different amounts of O precursors addition. Conditions: \({\varPhi }=0.5\); \({{ {P}}}_{1} =100 \, {\hbox {kPa}}\); and \({ {T}}_{1} =300 \, {\hbox {K}}\). Solid lines: neat DME; Long-dash lines: DME \(+\) 100 ppm additive; Short-dash lines: DME \(+\) 1000 ppm additive; Long-short-dash lines: DME \(+\) 10,000 ppm additive
Appendix 2: Important elementary reactions
Table 3 lists the elementary reactions that were identified as important through the analysis of the EER, OH and \({\hbox {HO}}_{2}\) ROP, and sensitivity coefficient on temperature. The number of the reaction corresponds to the one used in the text.
Rights and permissions
About this article
Cite this article
Mével, R., He, Y.Z. Effect of oxygen atom precursors addition on LTC-affected detonation in \({\hbox {DME}}{-}{\hbox {O}}_{2}{-}{\hbox {CO}}_{2}\) mixtures. Shock Waves 30, 799–807 (2020). https://doi.org/10.1007/s00193-020-00953-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00193-020-00953-0