Abstract
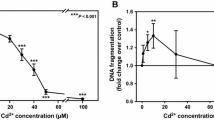
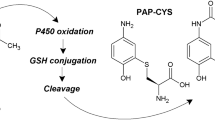
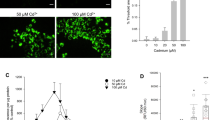
Proximal tubular cells from human (HPT) and rat (RPT) kidneys were isolated, grown to confluence and incubated with S-(1,2-dichlorovinyl)-l-cysteine (DCVC), S-(1,2,2-trichlorovinyl)-l-cysteine (TCVC), S-(1,1,2,2-tetrafluoroethyl)-l-cysteine (TFEC) and S-(2-chloro-1,1-difluorethyl)-l-cysteine (CDFEC), the cysteine conjugates of nephrotoxicants. The cultures were exposed to the conjugates for 12, 24 and 48 h and the toxicity determined using the MTT assay. All four conjugates caused dose-dependent toxicity to RPT cells over the range 50–1,000 μM, the order of toxicity being DCVC>TCVC>TFEC=CDFEC. The inclusion of aminooxyacetic acid (AOAA; 250 μM), an inhibitor of pyridoxal phosphate-dependent enzymes such as C-S lyase, afforded protection, indicating that C-S lyase has a role in the bioactivation of these conjugates. In HPT cultures only DCVC caused significant time- and dose-dependent toxicity. Exposure to DCVC (500 μM) for 48 h decreased cell viability to 7% of control cell values, whereas co-incubation of DCVC (500 μM) with AOAA (250 μM) resulted in cell viability of 71%. Human cultures were also exposed to S-(1,2-dichlorovinyl)-glutathione (DCVG). DCVG was toxic to HPT cells, but the onset of toxicity was delayed compared with the corresponding cysteine conjugate. AOAA afforded almost complete protection from DCVG toxicity. Acivicin (250 μM), an inhibitor of γ-glutamyl transferase (γ-GT), partially protected against DCVG (500 μM)-induced toxicity at 48 h (5% viability and 53% viability in the absence and presence of acivicin, respectively). These results suggest that DCVG requires processing by γ-GT prior to bioactivation by C-S lyase in HPT cells. The activity of C-S lyase, using TFEC as a substrate, and glutamine transaminase K (GTK) was measured in rat and human cells with time in culture. C-S lyase activity in RPT and HPT cells decreased to approximately 30% of fresh cell values by the time the cells reached confluence (120 h), whereas the decline in GTK activity was less marked (50% of the fresh cell values at confluence). Rat cells had threefold higher activity than human cells at each time point. This higher activity may partly explain the differences in toxicity between rat and human proximal tubular cells in culture.








Similar content being viewed by others
References
Anders MW, Dekant W (1998) Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol 38:501–537
Anders MW, Lash LH, Dekant W, Elfarra AA, Dohn DR (1988) Biosynthesis and biotransformation of glutathione S-conjugates to toxic metabolites. Crit Rev Toxicol 18:311–341
Birner G, Vamvakas S, Dekant W, Henschler D (1993) Nephrotoxic and genotoxic N-acetyl-S-dichlorovinyl-l-cysteine is a urinary metabolite after occupational 1,1,2-trichloroethene exposure in humans: implications for the risk of trichloroethene exposure. Environ Health Perspect 99:281–284
Bladeren PJ van (1988) Formation of toxic metabolites from drugs and other xenobiotics by glutathione conjugation. Trends Pharmacol Sci 9:295–299
Boogaard P, Commandeur JNM, Mulder GJ, Vermeulen NPE, Nagelkerke JF (1989) Toxicity of the cysteine-S-conjugates and mercapturic acids of four structurally related difluoroethylenes in isolated proximal tubular cells from rat kidney. Biochem Pharmacol 38:3731–3741
Chakrabarti S, Malick MA, Denniel C, Greselin E (1992) Species differences in the nephrotoxic response to S-(1,2-dichlorovinyl)glutathione. Toxicol Lett 60:343–351
Chen JC, Stevens JL, Trifillis AL, Jones TW (1990) Renal cysteine conjugate β-lyase-mediated toxicity studied with primary cultures of human proximal tubular cells. Toxicol Appl Pharmacol 103:463–473
Cooper AJL (1998) Mechanisms of cysteine S-conjugate β-lyases. Adv Enzymol 72A:199–238
Dohn DR, Leininger JR, Lash LH, Quebbemann AJ, Anders MW (1985) Nephrotoxicity of S-(2-chloro-1,1,2-trifluoroethyl)-glutathione and S-(2-chloro-1,1,2-trifluoro-ethyl)-l-cysteine, the glutathione and cysteine conjugates of chloro-trifluoroethene. J Pharmacol Exp Ther 235:851–857
Elfarra AA, Jakobson I, Anders MW (1986) Mechanism of S-(1,2-dichlorovinyl)-glutathione-induced nephrotoxicity. Biochem Pharmacol 35:283–283
Gordon EM, Whiting PH, Simpson JG, Hawksworth GM (1987) Isolation and characterisation of rat renal proximal tubular cells. Biochem Soc Trans 15:457–458
Green T, Odum J (1984) The metabolism and nephrotoxicity of tetrafluoroethylene in the rat. Toxicol Appl Pharmacol 76:306–318
Green T, Dow J, Ellis MK, Foster JR, Odum J (1997) The role of glutathione conjugation in the development of kidney tumours in rats exposed to trichloroethylene. Chem Biol Interact 105:99–117
Ishmael J, Lock EA (1986) Nephrotoxicity of hexachlorobutadiene and its glutathione-derived conjugates. Toxicol Pathol 14:258–262
Jones TW, Qin C, Schaeffer VH, Stevens JL (1988) Immunohistochemical localization of glutamine transaminase K, a rat kidney cysteine conjugate beta-lyase, and the relationship to the segment specificity of cysteine conjugate nephrotoxicity. Mol Pharmacol 34:621–627
Kim HS, Cha SH, Abraham DG, Cooper AJL, Endou H (1997) Intranephron distribution of cysteine S-conjugate beta-lyase activity and its implication for hexachloro-1,3-butadiene induced nephrotoxicity in rats. Arch Toxicol 71:131–141
Lash LH, Qian W, Putt DA, Desai K, Elfarra AA, Sicuri AR, Parker JC (1998) Glutathione conjugation of perchloroethylene in rats and mice in vitro: sex-, species-, and tissue-dependent differences. Toxicol Appl Pharmacol 150:49–57
Lock EA (1988) Studies on the mechanism of nephrotoxicity and nephrocarcinogenicity of halogenated alkenes. Crit Rev Toxicol 19:23–42
Lock EA, Ishmael J (1985) Effect of the organic acid transport inhibitor probenicid on renal cortical uptake and proximal tubular toxicity of hexachloro-1,3-butadiene and its conjugates. Toxicol Appl Pharmacol 8:32–42
MacFarlane M, Foster JR, Gibson GG, King LJ, Lock EA (1989) Cysteine conjugate β-lyase of rat kidney cytosol: characterization, immunocytochemical localization, and correlation with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol 98:185–197
McCarthy RI, Lock EA, Hawksworth GM (1994) Cytosolic C-S lyase activity in human kidney samples: relevance for the nephrotoxicity of halogenated alkenes in man. Toxicol Ind Health 10:103–112
McLaren J, Whiting P, Simpson J, Hawksworth G (1995) Isolation and characterisation of human proximal tubular cells derived from kidney cortical segments. Hum Exp Toxicol 14:916–922
Moore RB, Green T (1988) The synthesis of nephrotoxic conjugates of glutathione and cysteine. Toxicol Environ Chem 17:152–162
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Rodilla V, Hawksworth GM (1996) In: Jones GE (ed) Methods in molecular biology, vol XX. Human cell culture protocols. Humana, Totowa, NJ
Schaaf GJ, Groene EM de, Maas RF, Commandeur JNM, Fink-Gremmels J (2001) Characterisation of biotransformation enzyme activities in primary rat proximal tubular cells. Chem Biol Interact 134:167–190
Stevens JL, Hayden P, Taylor G (1986) The role of cysteine conjugate β-lyase in the mechanism of S-cysteine conjugate toxicity in LLC-PK1 cells. J Biol Chem 261:3325–3332
Stevens JL, Hatzinger PB, Hayden PJ (1989) Quantitation of multiple pathways for the metabolism of nephrotoxic cysteine conjugates using selective inhibitors of l-alpha-hydroxy acid oxidase (l-amino acid oxidase) and cysteine conjugate beta-lyase. Drug Metab Dispos 17:297–303
Trifillis AL, Regec AL, Trump BF (1985) Isolation, culture and characterization of human renal tubular cells. J Urol 133:324–329
Wang Z, Zaitsu K, Ohkura Y (1988) High performance liquid chromatographic determination of α-keto acids in human serum and urine using 1,2-diamino-4,5-methylenedioxybenzene as a precolumn fluorescence derivatization reagent. J Chromatogr 430:223–231
Wolfgang GHI, Gandolfi AJ, Stevens JL, Brendel K (1989) In vitro and in vivo nephrotoxicity of the l and d isomers of S-(1,2-dichlorovinyl)-cysteine. Toxicology 58:33–42
Wolfgang GHI, Gandolfi AJ, Nagle RB, Brendel K, Stevens JL (1990) Assessment of S-(1,2-dichlorovinyl)-l-cysteine induced toxic events in rabbit renal cortical slices. Biochemical and histological evaluation of uptake covalent binding and toxicity. Chem Biol Interact 75:153–170
Acknowledgements
Trevor McGoldrick was in receipt of a Wellcome Trust Toxicology Studentship. The experiments comply with current UK legislation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGoldrick, T.A., Lock, E.A., Rodilla, V. et al. Renal cysteine conjugate C-S lyase mediated toxicity of halogenated alkenes in primary cultures of human and rat proximal tubular cells. Arch Toxicol 77, 365–370 (2003). https://doi.org/10.1007/s00204-003-0459-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0459-6