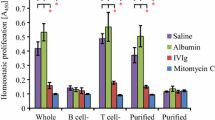
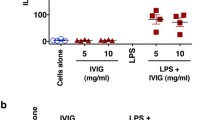
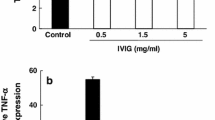
Abstract
Intravenous immunoglobulin (IVIG) has been used for the treatment of inflammatory and autoimmune diseases. The ability to modulate cytokine production has been formerly described as one of the mechanisms of its action. This study aimed to investigate the effect of IVIG on the production of pro-inflammatory cytokines in lipopolysaccharide (LPS)-stimulated monocytic cells. Peripheral blood mononuclear cells (PBMCs) or THP-1 cells treated with phorbol myristate acetate (PMA) were stimulated with LPS. The protein levels of pro-inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, and high-mobility group box 1 (HMGB1)] in the culture supernatants were determined using appropriate enzyme-linked immunosorbent assay kits. The mRNA of TNF-α was determined by reverse transcription–polymerase chain reaction. The phosphorylation of nuclear factor kappa B (NF-κB) and the mitogen-activated protein kinases was examined by Western blot analyses. IVIG suppressed the production of pro-inflammatory cytokines such as TNF-α and IL-6 in LPS-stimulated PBMCs. Furthermore, IVIG inhibited TNF-α, IL-6, and HMGB1 production from LPS-stimulated THP-1 cells treated with PMA. In addition, Fc fragment prepared from the IVIG inhibited production of these cytokines from the cells to the same degree as IVIG, whereas Fab and F(ab')2 fragments inhibited this only partially. We showed that IVIG and Fc fragments suppressed LPS-induced signal transduction pathways involving phosphorylation of NF-κB, p38, and c-Jun N-terminal kinase (JNK). Taken together, our results suggest that IVIG attenuates LPS-induced cytokine production predominantly mediated by its Fc region. The activity might be regulated by inhibiting NF-κB, p38, and JNK pathways in human monocytic cells.





Similar content being viewed by others
References
Abe Y, Horiuchi A, Miyake M, Kimura S (1994) Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev 139:5–19
Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101:36–47
Andersson U, Bjork L, Skansen-Saphir U, Andersson J (1994) Pooled human IgG modulates cytokine production in lymphocytes and monocytes. Immunol Rev 139:21–42
Campbell DE, Georgiou GM, Kemp AS (1999) Pooled human immunoglobulin inhibits IL-4 but not IFN-gamma or TNF-alpha secretion following in vitro stimulation of mononuclear cells with staphylococcal superantigen. Cytokine 11:359–365
Furukawa S, Matsubara T, Yone K, Hirano Y, Okumura K, Yabuta K (1992) Kawasaki disease fifers from anaphylactoid purpura and measles with regard to tumour necrosis factor-α and interleukin 6 in serum. Eur J Pediatr 151:44–47
Furusho K, Nakano H, Shinomiya K, Tamura T, Manabe Y, Kawarano M, Baba K, Kamiya T, Kiyosawa N, Hayashidera T, Hirose O, Yokoyama T, Baba K, Mori C (1984) High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 2:1055–1058
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T (2008) High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med 34:1812–1819
Ichiyama T, Ueno Y, Hasegawa M, Niimi A, Matsubara T, Furukawa S (2004) Intravenous immunoglobulin inhibits NF-κB activation and affects Fcγ receptor expression in monocytes/macrophages. Naunyn-Schmied Arch Pharmacol 369:428–433
Iwata M, Shimozato T, Tokiwa H, Tsubura E (1987) Antipyretic activity of a human immunoglobulin preparation for intravenous use in an experimental model of fever in rabbits. Infect Immun 55:547–554
Kazatchkine MD, Kaveri SV (2001) Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345:747–755
Kreymann KG, de Heer G, Nierhaus A, Kluge S (2007) Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 35:2677–2685
Laupland KB, Kirkpatrick AW, Delaney A (2007) Polyclonal intravenous immunoglobulins for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 35:2686–2692
Li S, Zhang J (2009) Lipopolysaccharide induces autotoxin expression in human monocytic THP-1 cells. Biochem Biophys Res Commun 378:264–268
Lin CY, Lin CC, Hwang B, Chiang B (1992) Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor α among patients with Kawasaki disease. J Pediatr 121:924–926
Maury CPJ, Salo E, Pelkonen P (1988) Circulating interleukin-1β in patients with Kawasaki disease. N Engl J Med 319:1670–1671
Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV (2007) Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol 27:233–245
Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Fulton DR, Lewis AB, Leung DYM, Colton T, Rosen FS, Melish ME (1986) The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 315:341–347
Oberholzer A, Oberholzer C, Moldawer LL (2001) Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16:83–96
Park EK, Jung HS, Yang HI, Yoo MC, Kim KS (2007) Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res 56:45–50
Parr D, Connell G, Kells D, Hofmann T (1976) Fb'2, a new peptic fragment of human immunoglobulin G. Biochem J 155:31–36
Pollack M (1983) Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in human. J Infect Dis 147:1090–1098
Rhoades CJ, Williams MA, Kelsey SM, Newland AC (2000) Monocyte-macrophage system as targets for immunomodulation by intravenous immunoglobulin. Blood Rev 14:14–30
Saito T, Kimura S, Tateda K, Mori N, Hosono N, Hayakawa K, Akasaka Y, Ishii T, Sumiyama Y, Kusachi S, Nagao J, Yamaguchi K (2011) Evidence of intravenous immunoglobulin as a critical supportive therapy against Clostridium difficile toxin-mediated lethality in mice. J Antimicrob Chemother 66:1096–1099
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND (2007) Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol 8:1–17
Shimozato T, Iwata M, Kawada TN (1991) Human immunoglobulin preparation for intravenous use induces elevation of cellular cyclic adenosine 3':5'-monophosphate levels, resulting in suppression of tumour necrosis factor alpha and interleukin-1 production. Immunology 72:497–501
Snigurowicz J, Powiertowska-Rezmer M (1980) Papain hydrolysis products in four M-IgG subclass. Arch Immunol Ther Exp 28:265–273
Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ (2006) The role of high mobility group box-1 protein in severe sepsis. Curr Opin Infect Dis 19:231–236
Takata Y, Seki S, Dobashi H, Takeshita S, Nakatani K, Kamezawa Y, Hiraide H, Sekine I, Yoshioka S (1998) Inhibition of IL-12 synthesis of peripheral blood mononuclear cells (PBMC) stimulated with a bacterial superantigen by pooled human immunoglobulin: implications for its effect on Kawasaki disease (KD). Clin Exp Immunol 114:311–319
Toungouz M, Denys CH, Groote DD, Dupont E (1995) In vitro inhibition of tumour necrosis factor-α and interleukin-6 production by intravenous immunoglobulins. Br J Haematol 89:698–703
Ulloa L, Tracey KJ (2005) The ‘cytokine profile’: a code for sepsis. Trends Mol Med 11:56–63
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Wu KH, Wu WM, Lu MY, Chiang BL (2006) Inhibitory effect of pooled human immunoglobulin on cytokine production in peripheral blood mononuclear cells. Pediatr Allergy Immunol 17:60–68
Wu W, Alexis NE, Chen X, Bromberg PA, Peden DB (2008) Involvement of mitogen-activated protein kinases and NF-κB in LPS-induced CD40 expression on human monocytic cells. Toxicol Appl Pharmacol 228:135–143
Yanagisawa C, Hanaki H, Natae T, Sunakawa K (2007) Neutralization of staphylococcal exotoxins in vitro by human-origin intravenous immunoglobulin. J Infect Chemother 13:368–372
Yang H, Tracey KJ (2010) Targeting HMGB1 in inflammation. Biochim Biophys Acta 1799:149–156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murakami, K., Suzuki, C., Kobayashi, F. et al. Intravenous immunoglobulin preparation attenuates LPS-induced production of pro-inflammatory cytokines in human monocytic cells by modulating TLR4-mediated signaling pathways. Naunyn-Schmiedeberg's Arch Pharmacol 385, 891–898 (2012). https://doi.org/10.1007/s00210-012-0765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-012-0765-8