Abstract
Rationale
Schizophrenia is a severe, disabling chronic disorder affecting approximately 1% of the population. Improvements and development of more robust and hopefully predictive screening assays for this disease should enhance the identification and development of novel treatments. The present study describes a rapid and robust method for the testing of potential novel antipsychotics by utilising a simplified [14C]2-deoxyglucose (2-DG) autoradiography method following memantine-induced brain activation.
Methods
Male C57BL/6JCRL mice were given vehicle, ketamine or memantine (10, 20 and 30 mg/kg, subcutaneously (s.c.)) and sacrificed 45 min post-[14 C]2-DG administration. In subsequent reversal studies, the memantine challenge was further validated with haloperidol (0.32 mg/kg, s.c.) and clozapine (2.5 and 10 mg/kg, s.c.) in parallel with the ketamine model (Duncan et al. 1998a). Lastly, the effects of an mGlu2/3 receptor agonist, LY404039 (10 mg/kg, s.c.), on both ketamine and memantine-induced brain activation was determined.
Results
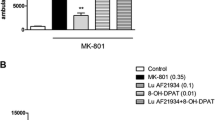
Both N-methyl-d-aspartate (NMDA) antagonists dose-dependently induced significant region-specific increases in 2-DG uptake. Interestingly, memantine elicited a considerably greater brain activation signature with a larger dynamic window than ketamine. The “atypical” antipsychotic clozapine significantly reversed memantine-induced 2-DG uptake whilst the “typical” antipsychotic haloperidol was inactive. Pre-treatment with LY404039 fully reversed both the ketamine- and memantine-induced increase in 2-DG uptake without effects on basal 2-DG uptake.
Conclusion
This novel pre-clinical imaging methodology displays potential for the screening of compounds targeting the NMDA receptor hypofunction hypothesis of schizophrenia and should assist in developing compounds from the bench to clinic.




Similar content being viewed by others
References
Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK (2010) Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res 88:143–154
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D (1997) Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 154:805–811
Brown ET, Umino Y, Loi T, Solessio E, Barlow R (2005) Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci 22(5):615–618
Colangelo V, Di GR, Passarelli F, Musicco M, Pontieri FE, Orzi F (1997) Differential effects of acute administration of clozapine or haloperidol on local cerebral glucose utilization in the rat. Brain Res 768:273–278
Conn PJ, Christopoulos A, Lindsley CW (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54
Dawson N, Ferrington L, Olverman HJ, Kelly PA (2008) Novel analysis for improved validity in semi-quantitative 2-deoxyglucose autoradiographic imaging. J Neurosci Methods 175:25–35
Depoortere R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, Poncelet M, Heaulme M, Santucci V, Decobert M, Cudennec A, Voltz C, Boulay D, Paul Terranova J, Stemmelin J, Roger P, Marabout B, Sevrin M, Vige X, Biton B, Steinberg R, Francon D, Alonso R, Avenet P, Oury-Donat F, Perrault G, Griebel G, George P, Soubrie P, Scatton B (2005) Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology 30:1963–1985
Duncan GE, Johnson KB, Breese GR (1993) Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neurosci 13:3932–3943
Duncan GE, Leipzig JN, Mailman RB, Lieberman JA (1998a) Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res 812:65–75
Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR (1998b) Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res 787:181–190
Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA (2000) Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alterations in regional brain metabolism. J Pharmacol Exp Ther 293:8–14
Eintrei C, Sokoloff L, Smith CB (1999) Effects of diazepam and ketamine administered individually or in combination on regional rates of glucose utilization in rat brain. Br J Anaesth 82:596–602
Gilling KE, Jatzke C, Hechenberger M, Parsons CG (2009) Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-d-aspartate (NMDA) channel blocker memantine at human NMDA (GluN1/GluN2A) receptors. Neuropharmacology 56:866–875
Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT, Loomis S, Mason R, Shahabi S, Tricklebank MD (2009) Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology (Berl) 205:203–216
Gleason SD, Shannon HE (1997) Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology 129:79–84
Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A (2007) Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology 33:1690–1703
Gunduz-Bruce H (2009) The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60:279–286
Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA (2005) Effects of noncompetitive nmda receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology 30:2275–2282
Huey ED, Dustin IH, Overman GP, Mirza N, Sunderland T (2005) Development of subtle psychotic symptoms with memantine: a case report. J Clin Psychiatry 66:658–659
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Kurumaji A, McCulloch J (1989) Effects of MK-801 upon local cerebral glucose utilisation in conscious rats and in rats anaesthetised with halothane. J Cereb Blood Flow Metab 9:786–794
Lahti AC, Holcomb HH, Medoff DR, Tamminga CA (1995) Ketamine activates psychosis and alters limbic blood flow in schizophrenia. NeuroReport 6:869–872
Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, Gage A (2009) A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology 34:1322–1329
Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams J, Sur C, Kinney GG (2006) Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem 6:771–785
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A (1996) NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14:301–307
Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ (2006) Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 316:315–324
Miller R (2009) Mechanisms of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions: part I. Curr Neuropharmacol 7:302–314
Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE (2000) Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology 22:400–412
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Olney JW, Newcomer JW, Farber NB (1991) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533
Parsons CG, Stoffler A, Danysz W (2007) Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system - too little activation is bad, too much is even worse. Neuropharmacology 53:699–723
Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13:1102–1107
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. Academic, San Diego
Riederer P, Lange KW, Kornhuber J, Danielczyk W (1991) Pharmacotoxic psychosis after memantine in Parkinson’s disease. Lancet 338:1022–1023
Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD (2007) In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 193:121–136
Schiffer WK, Mirrione MM, Dewey SL (2007) Optimizing experimental protocols for quantitative behavioral imaging with 18 F-FDG in rodents. J Nucl Med 48:277–287
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Spiegel D, Leader M (2007) Psychosis induced by the interaction of memantine and amantadine: lending evidence to the glutamatergic theory of schizophrenia. Clin Schizophr Relat Psychoses 1:273–276
Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J (1997a) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7:25–38
Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J (1997b) Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7:9–24
Warnock G, Moechars D, Langlois X, Steckler T (2009) In vivo evidence for ligand-specific receptor activation in the central CRF system, as measured by local cerebral glucose utilization. Peptides 30:947–954
Zeilhofer HU, Swandulla D, Geisslinger G, Brune K (1992) Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur J Pharmacol 213:155–158
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dedeurwaerdere, S., Wintmolders, C., Straetemans, R. et al. Memantine-induced brain activation as a model for the rapid screening of potential novel antipsychotic compounds: exemplified by activity of an mGlu2/3 receptor agonist. Psychopharmacology 214, 505–514 (2011). https://doi.org/10.1007/s00213-010-2052-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2052-z