Abstract
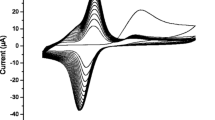
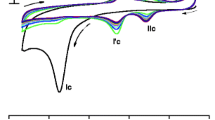
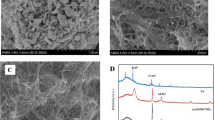
In this work, an electrochemical dihydronicotinamide adenine dinucleotide (NADH) sensor based on the catalytic growth of Au nanoparticles (Au NPs) on glassy carbon electrode was developed. Catalyzed by Au NPs immobilized on pretreated glassy carbon electrode, the reduction of AuCl4 − in the presence of hydroquinone and cetyltrimethyl ammonium chloride led to the formation of enlarged Au NPs on the electrode surface. Spectrophotometry and high-resolution scanning electronic microscope (SEM) analysis of the sensor morphologies before and after biocatalytic reaction revealed a diameter growth of the nanoparticles. The catalytic growth of Au NPs on electrode surface remarkably facilitated the electron transfer and improved the performance of the sensor. Under optimal conditions, NADH could be detected in the range from 1.25 × 10−6 to 3.08 × 10−4 M, and the detection limit was 2.5 × 10−7 M. The advantages of the proposed sensor, such as high precision and sensitivity, fast response, low cost, and good storage stability, made it suitable for on-line detection of NADH in complex biological systems and contaminant degradation processes.

Schematic presentation of the bioelectrocatalytic sensing of NADH







Similar content being viewed by others
References
Huang X, El-Sayed IH, Yi X, El-Sayed MA (2005) J Photochem Photobio B Biology 81:76–83
Álvarez-González MI, Saidman SB, Lobo-Castañón MJ, Miranda-Ordieres AJ, Tuñón-Blanco P (2000) Anal Chem 72:520–527
Gorton L, Dominguez E (2002) Rev Mol Biotech 82:371–392
Tang L, Zeng GM, Wang H, Shen GL, Huang DL (2005) Enzyme Microb Tech 36:960–966
Gros P, Comtat M (2004) Biosens Bioelectron 20:204–210
Jena BK, Raj CR (2006) Anal Chem 78:6332–6339
Manesh KM, Santhosh P, Gopalan A, Lee KP (2008) Talanta 75:1307–1314
Manso J, Mena ML, Yáñez-Sedeño P, Pingarrón JM (2008) Electrochim Acta 53:4007–4012
Tian F, Zhu G (2004) Sensor Actuat B 97:103–108
Valentini F, Salis A, Curulli A, Palleschi G (2004) Anal Chem 76:3244–3248
Welch CM, Compton RG (2006) Anal Bioanal Chem 384:601–619
Zhang Y, Zeng GM, Tang L, Huang DL, Jiang XY, Chen YN (2007) Biosens Bioelectron 22:2121–2126
Tang L, Zeng G, Liu J, Xu X, Zhang Y, Shen G, Li Y, Liu C (2008) Anal Bioanal Chem 391:679–685
Wang J, Musameh M (2003) Anal Chem 75:2075–2079
Banks CE, Compton RG (2005) Analyst 130:1232–1239
Yu A, Liang Z, Cho J, Caruso F (2003) Nano Lett 3:1203–1207
Wang J (2005) Small 1:1036–1043
Tang L, Zeng GM, Shen GL, Li YP, Zhang Y, Huang DL (2008) Environ Sci Technol 42:1207–1212
Mao X, Jiang J, Luo Y, Shen G, Yu R (2007) Talanta 73:420–424
Shlyahovsky B, Katz E, Xiao Y, Pavlov V, Willner I (2005) Small 1:213–216
Willner I, Basnar B, Willner B (2007) FEBS J 274:302–309
Zhao W, Ge PY, Xu JJ, Chen HY (2007) Langmuir 23:8597–8601
Zayats M, Baron R, Popov I, Willner I (2005) Nano Lett 5:21–25
Urban M, Möller R, Fritzsche W (2003) Rev Sci Instrum 74:1077–1081
Grubisha DS, Lipert RJ, Park HY, Driskell J, Porter MD (2003) Anal Chem 75:5936–5943
Xu SP, Ji XH, Xu WQ, Li XL, Wang LY, Bai YB, Zhao B, Ozaki Y (2004) Analyst 129:63–83
Xiao Y, Pavlov V, Levine S, Niazov T, Markovitch G, Willner I (2004) Angew Chem Int Ed 43:4519–4522
Willner I, Baron R, Willner B (2006) Adv Mater 18:1109–1120
Atchley SH, Clark JB (1979) Appl Environ Microb 38:1040–1044
Tarre S, Green M (2004) Appl Environ Microb 70:6481–6487
Radoi A, Compagnone D, Valcarcel MA, Placidi P, Materazzi S, Moscone D, Palleschi G (2008) Electrochim Acta 53:2161–2169
Gligor D, Balaj F, Maicaneanu A, Gropeanu R, Grosu I, Muresan L, Popescu IC (2008) Mater Chem Phys. doi:10.1016/j.matchemphys.2008.07.077 in press
Arroyo A, Kagan VE, Tyurin VA, Burgess JR, de Cabo R, Navas P, Villalba JM (2000) Antioxid Redox Sign 2:251–262
Musameh M, Wang J, Merkoci A, Lin Y (2002) Electrochem Commun 4:743–746
Chen J, Bao J, Cai C, Lu T (2004) Anal Chim Acta 516:29–34
Xiao L, Wildgoose GG, Compton RG (2008) Anal Chim Acta 620:44–49
Zeng G, Tang L, Shen G, Huang G, Niu C (2004) Int J Environ Anal Chem 84:761–774
Acknowledgments
The study was financially supported by the National Natural Science Foundation of China (No.50608029), the National 863 High Technology Research Program of China (No.2004AA649370, No.2006AA06Z407), the Chinese National Basic Research Program (973 Program; No.2005CB724203), the Natural Foundation for Distinguished Young Scholars (No.50425927, No.50225926), Program for Changjiang Scholars and Innovative Research Team in University (IRT0719) and the Hunan Provincial Natural Science Foundation of China (06JJ20062).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, L., Zeng, G., Shen, G. et al. Highly sensitive sensor for detection of NADH based on catalytic growth of Au nanoparticles on glassy carbon electrode. Anal Bioanal Chem 393, 1677–1684 (2009). https://doi.org/10.1007/s00216-008-2560-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2560-4