Abstract
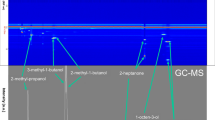
A new method for the growth-dependent headspace analysis of bacterial cultures by needle trap (NT)-gas chromatography-mass spectrometry (GC-MS) was established. NTs were used for the first time as enrichment technique for volatile organic compounds (VOCs) in the headspace of laboratory cultures. Reference strains of Escherichia coli and Pseudomonas aeruginosa were grown in different liquid culture media for 48 h at 36 °C. In the course of growth, bacterial culture headspace was analysed by NT-GC-MS. In parallel, the abiotic release of volatile organic compounds (VOC) from nutrient media was investigated by the same method. By examination of microbial headspace samples in comparison with those of uninoculated media, it could be clearly differentiated between products and compounds which serve as substrates. Specific microbial metabolites were detected and quantified during the stationary growth phase. P. aeruginosa produced dimethyl sulfide (max. 125 μg L−1 < limits of quantification (LOQ)), 1-undecene (max. 164 μg L−1) and 2-nonanone (max. 200 μg L−1), whereas E. coli produced carbon disulfide, butanal and indole (max. 149 mg L−1). Both organisms produced isoprene.

MVOCs produced by P. aeruginosa and E. coli at T = 36 °C in autoclaved LB + TRP medium






Similar content being viewed by others
References
Chigor VN, Umoh VJ, Smith SI (2010) Occurrence of Escherichia coli O157 in a river used for fresh produce irrigation in Nigeria. Afr J Biotechnol 9:178–182
Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K (2001) Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 138:699–704
Chmiel JF, Konstan MW, Elborn JS (2013) Antibiotic and anti-inflammatory therapies for cystic fibrosis. Cold Spring Harb Perspect Med 3:a009779
Kunze N, Göpel J, Kuhns M, Jünger M, Quintel M, Perl T (2013) Detection and validation of volatile metabolic patterns over different strains of two human pathogenic bacteria during their growth in a complex medium using multi-capillary column-ion mobility spectrometry (MCC-IMS). Appl Microbiol Biotechnol 97:3665–3676
Marcinowska R, Trygg J, Wolf-Watz H, Mortiz T, Surowiec I (2011) Optimization of a sample preparation method for the metabolomic analysis of clinically relevant bacteria. J Microbiol Methods 87:24–31
Eckner KF (1998) Comparison of membrane filtration and multiple-tube fermentation by the Colilert and Enterolert methods for detection of waterborne coliform bacteria, Escherichia coli, and enterococci used in drinking and bathing water quality monitoring in southern Sweden. Appl Environ Microbiol 64:3079–3083
Rabis T, Sommerwerck U, Anhenn O, Darwiche K, Freitag L, Teschler H, Bödeker B, Maddula S, Baumbach J (2011) Detection of infectious agents in the airways by ion mobility spectrometry of exhaled breath. Int J Ion Mobil Spec 14:187–195
Taucher J, Hansel A, Jordan A, Fall R, Futrell JH, Lindinger W (1997) Detection of isoprene in expired air from human subjects using proton-transfer-reaction mass spectrometry. Rapid Commun Mass Spectrom 11:1230–1234
Scotter JM, Allardyce RA, Langford VS, Hill A, Murdoch DR (2006) The rapid evaluation of bacterial growth in blood cultures by selected ion flow tube-mass spectrometry (SIFT-MS) and comparison with the BacT/ALERT automated blood culture system. J Microbiol Methods 65:628–631
Labows JN, McGinley KJ, Webster GF, Leyden JJ (1980) Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography–mass spectrometry. J Clin Microbiol 12:521–526
Koek MM, Muilwijk B, Van DWMJ, Hankemeier T (2006) Microbial metabolomics with gas chromatography / mass spectrometry. Anal Chem 78:1272–1281
Shestivska V, Spanel P, Dryahina K, Sovova K, Smith D, Musilek M, Nemec A (2012) Variability in the concentrations of volatile metabolites emitted by genotypically different strains of Pseudomonas aeruginosa. J Appl Microbiol 113:701–713
Boots AW, van Berkel JJBN, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ (2012) The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res 6:027108/027101–027121
Dummer J, Storer M, Swanney M, McEwan M, Scott-Thomas A, Bhandari S, Chambers S, Dweik R, Epton M (2011) Analysis of biogenic volatile organic compounds in human health and disease. TrAC Trends Anal Chem 30:960–967
Chambers ST, Syhre M, Murdoch DR, McCartin F, Epton MJ (2009) Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med Mycol 47:468–476
Scott-Thomas AJ, Syhre M, Pattemore PK, Epton M, Laing R, Pearson J, Chambers ST (2010) 2-aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med 10:1–10
Bean HD, Dimandja J-MD, Hill JE (2012) Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr B 901:41–46
Pawliszyn JB (2001) Needle trap. United States Patent US20010032521A1, Oct. 25, 2001
Trefz P, Kischkel S, Hein D, James ES, Schubert JK, Miekisch W (2012) Needle trap micro-extraction for VOC analysis: effects of packing materials and desorption parameters. J Chromatogr A 1219:29–38
Mieth M, Kischkel S, Schubert JK, Hein D, Miekisch W (2009) Multibed needle trap devices for on site sampling and preconcentration of volatile breath biomarkers. Anal Chem 81:5851–5857
Lord HL, Zhan W, Pawliszyn J (2010) Fundamentals and applications of needle trap devices: a critical review. Anal Chim Acta 677:3–18
Laaks J, Jochmann MA, Schmidt TC (2013) Empfindlich, automatisch und ohne Lösungsmittel. Nachr Chem 61:54–56
Mieth M, Schubert JK, Groger T, Sabel B, Kischkel S, Fuchs P, Hein D, Zimmermann R, Miekisch W (2010) Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal Chem 82:2541–2551
Eom I-Y, Pawliszyn J (2008) Simple sample transfer technique by internally expanded desorptive flow for needle trap devices. J Sep Sci 31:2283–2287
Trefz P, Roesner L, Hein D, Schubert JK, Miekisch W (2013) Evaluation of needle trap micro-extraction and automatic alveolar sampling for point-of-care breath analysis. Anal Bioanal Chem 405:3105–3115
Bos LDJ, Sterk PJ, Schultz MJ (2013) Volatile metabolites of pathogens: a systematic review. PLoS Pathog 9:e1003311/1003311–1003318
Schoeller C, Molin S, Wilkins K (1997) Volatile metabolites from some gram-negative bacteria. Chemosphere 35:1487–1495
Storer MK, Hibbard-Melles K, Davis B, Scotter J (2011) Detection of volatile compounds produced by microbial growth in urine by selected ion flow tube mass spectrometry (SIFT-MS). J Microbiol Methods 87:111–113
Demirev PA, Ho Y-P, Ryzhov V, Fenselau C (1999) Microorganism identification by mass spectrometry and protein database searches. Anal Chem 71:2732–2738
Bunge M, Araghipour N, Mikoviny T, Dunkl J, Schnitzhofer R, Hansel A, Schinner F, Wisthaler A, Margesin R, Maerk TD (2008) On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry. Appl Environ Microbiol 74:2179–2186
Mann S (1966) Über den Geruchsstoff von Pseudomonas aeruginosa. Arch Microbiol 54:184–190
Wildeboer D, Amirat L, Price RG, Abuknesha RA (2010) Rapid detection of Escherichia coli in water using a hand-held fluorescence detector. Water Res 44:2621–2628
Cox CD, Parker J (1979) Use of 2-aminoacetophenone production in identification of Pseudomonas aeruginosa. J Clin Microbiol 9:479–484
Normenausschuss Materialprüfung (NMP) im DIN, chemische Analytik—Nachweis-, Erfassungs- und Bestimmungsgrenze unter Wiederholbedingungen—Begriffe, Verfahren, Auswertung. Deutsche Norm DIN 32645, DIN Deutsches Institut für Normung e.V., Berlin, Berlin (November 2008).
Jochmann MA, Kmiecik MP, Schmidt TC (2006) Solid-phase dynamic extraction for the enrichment of polar volatile organic compounds from water. J Chromatogr A 1115:208–216
Jochmann MA, Yuan X, Schmidt TC (2007) Determination of volatile organic hydrocarbons in water samples by solid-phase dynamic extraction. Anal Bioanal Chem 387:2163–2174
Sieg K, Fries E, Püttmann W (2008) Analysis of benzene, toluene, ethylbenzene, xylenes and n-aldehydes in melted snow water via solid-phase dynamic extraction combined with gas chromatography/mass spectrometry. J Chromatogr A 1178:178–186
Wu J, Tragas C, Lord H, Pawliszyn J (2002) Analysis of polar pesticides in water and wine samples by automated in-tube solid-phase microextraction coupled with high-performance liquid chromatography–mass spectrometry. J Chromatogr A 976:357–367
Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J, Amann A (2012) Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol 12:113
Juenger M, Vautz W, Kuhns M, Hofmann L, Ulbricht S, Baumbach JI, Quintel M, Perl T (2012) Ion mobility spectrometry for microbial volatile organic compounds: a new identification tool for human pathogenic bacteria. Appl Microbiol Biotechnol 93:2603–2614
Thorn RMS, Reynolds DM, Greenman J (2011) Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J Microbiol Methods 84:258–264
Zechman JM, Aldinger S, Labows JN Jr (1986) Characterization of pathogenic bacteria by automated headspace concentration—gas chromatography. J Chromatogr B 377:49–57
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Prod Rep 24:814–842
Kuzma J, Nemecek-Marshall M, Pollock WH, Fall R (1995) Bacteria produce the volatile hydrocarbon isoprene. Curr Microbiol 30:97–103
Allardyce RA, Hill AL, Murdoch DR (2006) The rapid evaluation of bacterial growth and antibiotic susceptibility in blood cultures by selected ion flow tube mass spectrometry. Diagn Microbiol Infect Dis 55:255–261
Zhu J, Bean HD, Kuo Y-M, Hill JE (2010) Fast detection of volatile organic compounds from bacterial cultures by secondary electrospray ionization-mass spectrometry. J Clin Microbiol 48:4426–4431
Umber BJ, Shin H-W, Meinardi S, Leu S-Y, Zaldivar F, Cooper DM, Blake DR (2013) Gas signatures from Escherichia coli and Escherichia coli-inoculated human whole blood. Clin Transl Med 2:13
Acknowledgments
The authors gratefully acknowledge PAS Technology Deutschland GmbH for providing the Needle Trap Devices and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zscheppank, C., Wiegand, H.L., Lenzen, C. et al. Investigation of volatile metabolites during growth of Escherichia coli and Pseudomonas aeruginosa by needle trap-GC-MS. Anal Bioanal Chem 406, 6617–6628 (2014). https://doi.org/10.1007/s00216-014-8111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8111-2