Abstract
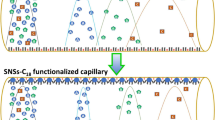
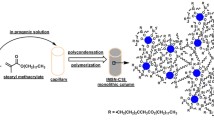
Cell membrane chromatography (CMC) is a powerful tool to study membrane protein interactions and to screen active compounds extracted from natural products. Unfortunately, a large amount of cells are typically required for column preparation in order to carry out analyses in an efficient manner. Micro-CMC (mCMC) has recently been developed by using a silica capillary as a membrane carrier. However, a reduced retention of analytes is generally associated with mCMC mostly due to a low ligand (cellular membrane) capacity. To solve this common problem, in this work a silica-based porous layer open tubular (PLOT) capillary was fabricated and, to the best of our knowledge, for the first time applied to mCMC. The mCMC column was prepared by physical adsorption of rabbit red blood cell (rRBC) membranes onto the inner surface of the PLOT capillary. The effects of the PLOT capillaries fabricated by different feed compositions, on the immobilization amount of cellular membranes (represented by the fluorescence intensity of the capillary immobilized with fluorescein isothiocyanate isomer-labeled cellular membranes) and on the dynamic binding capacity (DBC) of verapamil (VP, a widely used calcium antagonist which specific interacts with L-type calcium channel proteins located on cellular membrane of rRBC) have been systematically investigated. The fluorescence intensity of the mCMC column when combined with the PLOT capillary was found to be more than five times higher than the intensity using a bare capillary. This intriguing result indicates that the PLOT capillary exhibits a higher cellular membrane capacity. The DBC of VP in the PLOT column was found to be more than nine times higher than that in the bare capillary. An rRBC/CMC column was also prepared for comparative studies. As a result, mCMC provides similar chromatographic retention factors and stability with common CMC; however, the cellular membrane consumption for mCMC was found to be more than 460 times lower than that for CMC.

Comparision of mCMC chromatograms and SEM images between bare capillary and PLOT capillary



Similar content being viewed by others
References
Overington JP, AI-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev. 2006;5:993–6.
Kim HS, Kye YS, Hage DS. Development and evaluation of N-hydroxysuccinimide-activated silica for immobilizing human serum albumin in liquid chromatography columns. J Chromatogr A. 2004;1049:51–61.
Moaddel R, Marszałł MP, Bighi F, Yang Q, Duan X, Wainer IW. Automated ligand fishing using human serum albumin-coated magnetic beads. Anal Chem. 2007;79:5414–7.
Marszałł MP, Moaddel R, Kole S, Gandhari M, Bernier M, Wainer IW. Ligand and protein fishing with heat shock protein 90 coated magnetic beads. Anal Chem. 2008;80:7571–5.
Chen X, Cao Y, Zhang H, Zhu Z, Liu M, Liu H, et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Aconitum carmichaeli. Anal Chem. 2014;86:4748–57.
He L, Wang S, Geng X. Coating and fusing cell membranes onto a silica surface and their chromatographic characteristics. Chromatographia. 2001;54:71–6.
Wang S, Sun M, Zhang Y, Du H, He L. A new A431/cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening epidermal growth factor receptor antagonists from radix Sophorae flavescentis. J Chromatogr A. 2010;1217:5246–52.
Moaddel R, Wainer IW. The preparation and development of cellular membrane affinity chromatography columns. Nat Protoc. 2009;4:197–205.
Singh NS, Habicht K-L, Dossou KSS, Shimmo R, Wainer IW, Moaddel R. Multiple protein stationary phase: a review. J Chromatogr B. 2014;968:64–8.
Beigi F, Wainer IW. Syntheses of immobilized G protein-coupled receptor chromatographic stationary phases: characterization of immobilized μ and κ opioid receptors. Anal Chem. 2003;75:4480–5.
Beigi F, Chakir K, Xiao RP, Wainer IW. G-protein-coupled receptor chromatographic stationary phases. 2. Ligand-induced conformational mobility in an immobilized β2-adrenergic receptor. Anal Chem. 2004;76:7178–93.
Moaddel R, Jozwiak K, Whittington K, Wainer IW. Conformational mobility of immobilized α3β2, α3β4, α4β2 and α4β4 nicotinic acetylcholine receptors. Anal Chem. 2005;77:895–901.
Moaddel R, Jozwiak K, Yamaguchi R, Wainer IW. Direct chromatographic determination of dissociation rate constants of ligand-receptor complexes: assessment of the interaction of noncompetitive inhibitors with an immobilized nicotinic acetylcholine receptor-based liquid chromatography stationary phase. Anal Chem. 2005;77:5421–6.
Moaddel R, Oliveira RV, Kimura T, Hyppolite P, Juhaszova M, Xiao Y, et al. Initial synthesis and characterization of an α7 nicotinic receptor cellular membrane affinity chromatography column: effect of receptor subtype and cell type. Anal Chem. 2008;80:48–54.
Habicht KL, Frazier C, Singh N, Shimmo R, Wainer IW, Moaddel R. The synthesis and characterization of a nuclear membrane affinity chromatography column for the study of human breast cancer resistant protein (BCRP) using nuclear membranes obtained from the LN-229 cells. J Pharm Biomed Anal. 2013;72:159–62.
Li W, Niu X, Zhou P, Li M, He L. A combined peritoneal macrophage/cell membrane chromatography and offline GC-MS method for screening anti-inflammatory components from Chinese traditional medicine Houttuynia cordata Thumb. Chromatographia. 2011;73:673–80.
Sun M, Ran J, Du H, Zhang Y, Zhang J, Wang S, et al. A combined A431 cell membrane chromatography and online high performance liquid chromatography/mass spectrometry method for screening compounds from total alkaloid of Radix caulophylli acting on the human EGFR. J Chromatogr B. 2010;878:2712–8.
Hou X, Wang S, Zhang T, Ma J, Zhang J, Zhang Y, et al. Recent advances in cell membrane chromatography for traditional Chinese medicines analysis. J Pharm Biomed Anal. 2014;101:141–50.
Chen X, Cao Y, Lv D, Zhu Z, Zhang J, Chai Y. Comprehensive two-dimensional HepG2/cell membrane chromatography/monolithic column/time-of-flight mass spectrometry system for screening anti-tumor components from herbal medicines. J Chromatogr A. 2012;1242:67–74.
Moaddel R, Bullock PL, Wainer IW. Development and characterization of an open tubular column containing immobilized P-glycoprotein for rapid on-line screening for P-glycoprotein substrates. J Chromatogr B. 2004;799:255–63.
Du H, Ren J, Wang S, He L. Cell membrane chromatography competitive binding analysis for characterization of α1A adrenoreceptor binding interactions. Anal Bioanal Chem. 2011;400:3625–33.
He L, Wang S, Yang G, Zhang Y, Wang C, Yuan B, et al. Progress in cell membrane chromatography. Drug Discov Ther. 2007;1:104–7.
Poliakoff M, Licence P. Modern life depends on the petrochemical industry—most drugs, paints and plastics derive from oil. But current processes for making chemical products are not sustainable in terms of resources and environmental impact. Green chemistry aims to tackle this problem, and real progress is being made. Nature. 2007;450:810–2.
Tang C, Liu ZS, Qin N, Xu L, Duan HQ. Novel cell membrane capillary chromatography for screening active compounds from natural products. Chromatographia. 2013;76:697–701.
Gregg SJ, Sing KSW. Adsorption, surface area and porosity. 2nd ed. Academic Press Inc; 1982. p 132–153, 283–286.
Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. PNAS. 2011;108:10980–5.
Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–30.
Forster S, Kolmar H, Altmaier S. Synthesis and characterization of new generation open tubular silica capillaries for liquid chromatography. J Chromatogr A. 2012;1265:88–94.
Forster S, Kolmar H, Altmaier S. Preparation and kinetic performance assessment of thick film 10–12 μm open tubular silica capillaries in normal phase high pressure liquid chromatography. J Chromatogr A. 2013;1315:127–34.
Forster S, Kolmar H, Altmaier S. Performance evaluation of thick film open tubular capillary by reversed phase liquid chromatography. J Chromatogr A. 2013;1283:110–5.
Xu L, Cui P, Wang D, Tang C, Dong L, Zhang C, et al. Preparation and characterization of lysine-immobilized poly(glycidyl methacrylate) nanoparticle-coated capillary for the separation of amino acids by open tubular capillary electrochromatography. J Chromatogr A. 2014;1323:179–83.
Hermanson GT. Bioconjugate techniques, 2nd ed, Elsevier; 2008, p 170.
Rana TM, Meares CF. N-terminal modification of immunoglobulin polypeptide chains tagged with isothiocyanato chelates. Bioconjug Chem. 1990;1:357–62.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (No. 81402889 and No. 81303191) and the Natural Science Foundation of Tianjin (No. 14JCYBJC24300). We would also like to thank Prof. L. He and Prof. S. Wang at Xi’an Jiaotong University for their valuable guidance on this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical consent
The animal experiments have been approved by the Administrative Committee of Experimental Animal Care and Use of Tianjin Medical University and furthermore conformed to the guidelines set by the National Institute of Health on the ethical use of animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 556 kb)
Rights and permissions
About this article
Cite this article
Zhang, F., Zhao, X., Xu, B. et al. Preparation and characterization of micro-cell membrane chromatographic column with silica-based porous layer open tubular capillary as cellular membrane carrier. Anal Bioanal Chem 408, 2441–2448 (2016). https://doi.org/10.1007/s00216-016-9339-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9339-9