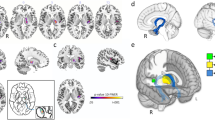
Abstract
Mirror apraxia is a condition in which patients with lesions of the posterior parietal cortex have deficits in reaching to objects presented through a mirror. The aim of the present study was to investigate possible mechanisms underlying this disorder. First, we addressed the question of whether mirror apraxia is exhibited to the same extent in peripersonal and in body space. Four patients with lesions of the posterior parietal lobe on either side and with marked mirror apraxia were required to reach for objects that were presented to them through a mirror and located either in body space (i.e. on the body surface) or in peripersonal space (i.e. in the reaching distance). Whereas reaching for objects located in body space was flawless in all patients, the performance deteriorated when the same objects were transferred to the peripersonal space. Although the objects were located only a few centimetres above the body surface, the patients reached towards the virtual object in the mirror. Based on these results we suggest that mirror apraxia may originate from a dissociation between the representations of body schema and peripersonal space and that objects located on the body surface become integrated into the body schema. In the second part of the study, using positron emission tomography study (PET), we studied the cerebral activation pattern during reaching to objects presented through a mirror in the peripersonal space in healthy subjects. The results show that increased neural activity in the anterior part of the intraparietal sulcus and in the dorsal premotor cortex was bound to the transformation of the target position from the mirror space to the real space. In contrast, the activity related to object localization in the mirror occurred at the parieto-occipital junction. Both mirror and arm transformation involved the medial posterior part of the superior parietal lobule, putatively area V6a. The results demonstrate that acting through a mirror is processed in a number of cortical areas of the dorsal stream.





Similar content being viewed by others
References
Binkofski F, Seitz RJ, Dohle C, Posse S, Schnitzler A, Müller-Gärtner HW, Jäncke L, Freund HJ (1997) Dissociation of retinotopic space and proprioceptive body scheme: characterization of a parietal cortex lesion. Soc Neurosci Abs 23:2374
Binkofski F, Buccino G, Dohle C, Seitz RJ, Freund H-J (1999) Mirror agnosia and mirror ataxia constitute different parietal lobe disorders. Ann Neurol 46:51–61
Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund H-J, Zilles K, Seitz RJ (2000) Broca's region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp11:273–285
Binkofski F, Kunesch E, Classen J, Seitz RJ, Freund H-J. (2001) Tactile apraxia: an unimodal disorder of tactile object exploration associated with parietal lesions. Brain 124:132–144
Bjoertomt O, Cowey A, Walsh V (2002) Spatial neglect in near and far space investigated by repetitive transcranial magnetic stimulation. Brain 125:2012–2022
Blanke O, Ortigue S, Landis T, Seeck M (2002) Stimulating illusory own-body perceptions. Nature 419:269–270
Bremmer F, Duhamel JR, BenHamed S, Graf W (1996) Non-retinocentric coding of visual space in the macaque ventral intraparietal area (VIP). Soc Neurosci Abstr 22:666.8
Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann KP, Zilles K, Fink G (2001) Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29:287–296
Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Reichle ME, van Essen DC et al. (1998) A common network of functional areas for attention and eye movements. Neuron 21:761–773
Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3:292–297
Culham JC, Kanwisher NG (2001) Neuroimaging of cognitive functions in humans. Curr Opin Neurobiol 11:157–163
Duhamel JR, Bremmer F, BenHamed S, Graf W (1997) Spatial invariance of visual receptive fields in parietal cortex neurons. Nature 389:845–848
Fattori P, Gamberoni M, Kutz DF, Galletti C (2001) "Arm reaching" neurons in the parietal area V6A of the macaque monkey. Eur J Neurosci 13:2309–2313
Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ (1996) Where in the brain does visual attention select the forest and the trees? Nature 382:626–628
Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M and Rizzolatti G (1996) Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol 76:141–157
Freund H-J (1987) Abnormalities of motor behavior after cortical lesions in man. In: Plum F (ed) Handbook of physiology. Vol 5, the nervous system: higher functions of the brain. Williams and Wilkins, Baltimore, pp 763–810
Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R (1995a) Spatial registration and normalization of images. Hum Brain Mapp 3:165–189
Friston K, Holmes A, Worsley K, Poline J-B, Frith C, Frackowiak R (1995b) Statistical parametric maps in functional imaging: general linear approach. Hum Brain Mapp 2:189–210
Friston KJ, Holmes AP, Price CJ, Büche C, Worsley KJ (1999) Multisubject MRI studies and conjunction analyses. Neuroimage 10:385–396
Galetti C, Battaglini PP, Fattori P (1991) Functional properties of neurons in the anterior bank of the parieto-occipital sulcus of the macaque monkey. Eur J Neurosci 3:452–461
Galetti C, Fattori P, Battaglini PP, Shipp S, Zeki S (1996) Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the macaque monkey. Eur J Neurosci 8:30–52
Gallup GG (1970) Chimpanzees: self-recognition. Science 167:86–87
Graziano MSA, Yap GS, Gross CG (1994) Coding of visual space by premotor neurons. Science 266:1054–1057
Iriki A, Tanaka M, Iwamura Y (1996) Coding of modified body schema during tool use by macaque postcentral neurons. Neuroreport 7:2325–2330
Liepmann H (1920) Apraxie: Brusch's Ergebnisse der Gesamten Medizin. Urban & Schwarzenberg, Berlin, pp 518–543
Matelli M, Luppino G, Murata A, Sakata H (1994) Independent anatomical circuits for reaching and grasping linking inferior parietal lobule and inferior area 6 in the monkey. Soc Neurosci Abstr 20:404.4
Milner AD, Goodale MA (1993) Visual pathways to perception and action. Prog Brain Res 95:317–338
Owen AM, Doyon J, Petrides M, Evans AC (1996) Planning and spatial working memory: a position emission tomography study in humans. Eur J Neurosci 8:353–364
Perenin MT, Vighetto A (1988) Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain 111:643–674
Petrides M, Pandya DN (1984) Projections of the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol 228:105–116
Ramachandran VS, Altschuler EL, Hillyer S (1997) Mirror agnosia. Proc R Soc Lond B Biol Sci 264:645–647
Rizzolatti G, Matelli M (2003) Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res (this issue)
Rizzolatti G, Luppino G, Matelli M (1998) The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106:283–296
Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000) The prefrontal cortex: response selection or maintenance within working memory? Science 288:1656–1660
Rushworth MF, Paus T, Sipila PH (2001) Attention systems and the organization of the human parietal cortex. J Neurosci 21:5262–5271
Seitz RJ, Binkofski F (2003) Modular organization of parietal lobe function as revealed by functional activation studies. In: Siegel AM, Andersen RA, Freund H-J, Spencer DD (eds) Advances in neurology: the parietal lobes. Lippincott Williams & Wilkins, Philadelphia, pp 281–292
Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S (2002) Topographical layout of hand, eye, calculation, and language-related areas in the human posterior parietal lobe. Neuron 33:475–487
Snyder LH, Grieve KL, Brotchie P, Andersen RA (1998) Separate body- and world-referenced representations of visual space in parietal cortex. Nature 294:887–891
Talairach J, Tournoux P (1988) Co-planar stereotactic atlas of the human brain. Thieme-Verlag, New York
Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM (1997) Functional analysis of V3A and related areas in human visual cortex. J Neurosci 17:7060–7078
Ungerleider L, Mishkin M (1982) Two cortical visual systems. In: Analysis of visual behavior. Ingle DJ, Goodale M, Mansfield RJW (eds) MIT Press, Cambridge, pp 549–586
van den Bos E, Jeannerod M. (2002) Sense of body and sense of action both contribute to self-recognition. Cognition 85:177–187
Van Essen DC, Zeki SM (1978) The topographic organization of rhesus monkey prestriate cortex. J Physiol (Lond) 277:193–226
Weiss PH, Marshall JC, Wunderlich G, Tellmann L et al. (2000) Neural consequences of action in near versus far space: a physiological basis for clinical dissociations. Brain 123:2531–2541
Wojciulik E, Kanwisher N (1999) The generality of parietal involvement in visual attention. Neuron 23:747–764
Acknowledgements
This study was supported by grants from: Deutsche Forschungsgemeinschaft SFB194, Volkswagenstiftung I-79006, BMBF GFGO 1337400.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binkofski, F., Butler, A., Buccino, G. et al. Mirror apraxia affects the peripersonal mirror space. A combined lesion and cerebral activation study. Exp Brain Res 153, 210–219 (2003). https://doi.org/10.1007/s00221-003-1594-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1594-2