Abstract
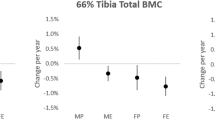
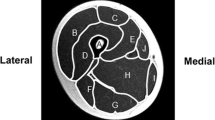
High impact loading is known to prevent some of the age-related bone loss but its effects on the density distribution of cortical bone are relatively unknown. This study examined the effects of age and habitual sprinting on tibial and fibular mid-shaft bone traits (structural, cortical radial and polar bone mineral density distributions). Data from 67 habitual male sprinters aged 19–39 and 65–84 years, and 60 non-athletic men (referents) aged 21–39 and 65–80 years are reported. Tibial and fibular mid-shaft bone traits (strength strain index SSI, cortical density CoD, and polar and radial cortical density distributions) were assessed with peripheral quantitative computed tomography. Analysis of covariance (ANCOVA) adjusted for height and body mass indicated that the sprinters had 21 % greater tibial SSI (P < 0.001) compared to the referents, with no group × age-group interaction (P = 0.54). At the fibula no group difference or group × age-group interaction was identified (P = 0.12–0.81). For tibial radial density distribution ANCOVA indicated no group × radial division (P = 0.50) or group × age-group × division interaction (P = 0.63), whereas an age × radial division interaction was observed (P < 0.001). For polar density distribution, no age-group × polar sector (P = 0.21), group × polar sector (P = 0.46), or group × age-group × polar sector interactions were detected (P = 0.15). Habitual sprint training appears to maintain tibial bone strength, but not radial cortical density distribution into older age. Fibular bone strength appeared unaffected by habitual sprinting.





Similar content being viewed by others
References
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. The Lancet 359(9319):1761–1767. doi:10.1016/S0140-6736(02)08657-9
Keaveny TM, Kopperdahl DL, Melton LJ et al (2010) Age-dependence of femoral strength in white women and men. J Bone Miner Res 25(5):994–1001
Karinkanta S, Piirtola M, Sievänen H et al (2010) Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol 6(7):396–407. doi:10.1038/nrendo.2010.70
Asikainen T-M, Kukkonen-Harjula K, Miilunpalo S (2004) Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med Auckl NZ 34(11):753–778
Nikander R, Sievanen H, Heinonen A et al (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 8(1):47. doi:10.1186/1741-7015-8-47
Wilks DC, Winwood K, Gilliver SF et al (2009) Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone 45(1):91–97. doi:10.1016/j.bone.2009.03.660
Weidauer LA, Eilers MM, Binkley TL et al (2012) Effect of different collegiate sports on cortical bone in the tibia. J Musculoskelet Neuronal Interact 12(2):68–73
Rantalainen T, Nikander R, Heinonen A et al (2010) Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: a pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int 86(6):447–454. doi:10.1007/s00223-010-9358-z
Marchi D, Shaw CN (2011) Variation in fibular robusticity reflects variation in mobility patterns. J Hum Evol 61(5):609–616. doi:10.1016/j.jhevol.2011.08.005
Mikkola TM, Sipilä S, Rantanen T et al (2008) Genetic and environmental influence on structural strength of weight-bearing and non–weight-bearing bone: a twin study. J Bone Miner Res 23(4):492–498. doi:10.1359/jbmr.071205
Mikkola TM, Sipilä S, Rantanen T et al (2009) Muscle cross-sectional area and structural bone strength share genetic and environmental effects in older women. J Bone Miner Res 24(2):338–345. doi:10.1359/jbmr.081008
Ma H, Leskinen T, Alen M et al (2009) Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res 24(8):1427–1433. doi:10.1359/jbmr.090309
Macdonald HM, Cooper DML, McKay HA (2008) Anterior–posterior bending strength at the tibial shaft increases with physical activity in boys: evidence for non-uniform geometric adaptation. Osteoporos Int 20(1):61–70. doi:10.1007/s00198-008-0636-9
Leppanen OV, Sievanen H, Jokihaara J et al (2010) The effects of loading and estrogen on rat bone growth. J Appl Physiol 108(6):1737–1744. doi:10.1152/japplphysiol.00989.2009
Cheng S, Sipilä S, Taaffe DR et al (2002) Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women. Bone 31(1):126–135
Rantalainen T, Nikander R, Daly RM et al (2011) Exercise loading and cortical bone distribution at the tibial shaft. Bone 48(4):786–791. doi:10.1016/j.bone.2010.11.013
Rantalainen T, Nikander R, Heinonen A et al (2011) An open source approach for regional cortical bone mineral density analysis. J Musculoskelet Neuronal Interact 11(3):243–248
Feik SA, Thomas CDL, Bruns R, Clement JG (2000) Regional variations in cortical modeling in the femoral mid-shaft: sex and age differences. Am J Phys Anthropol 112(2):191–205
Bell KL, Loveridge N, Power J et al (1999) Regional differences in cortical porosity in the fractured femoral neck. Bone 24(1):57–64
Zebaze R, Ghasem-Zadeh A, Bohte A et al (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. The Lancet 375(9727):1729–1736
Atkinson PJ, Weatherell JA (1967) Variation in the density of the femoral diaphysis with age. J Bone Joint Surg Br 49:781–788
Carballido-Gamio J, Harnish R, Saeed I et al (2013) Proximal femoral density distribution and structure in relation to age and hip fracture risk in women. J Bone Miner Res 28(3):537–546
Seeman E (1998) Growth in bone mass and size—are racial and gender differences in bone mineral density more apparent than real? J Clin Endocrinol Metab 83(5):1414–1419. doi:10.1210/jc.83.5.1414
Bousson V, Bergot C, Meunier A et al (2000) CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity1. Radiology 217(1):179–187
Bailey CA, Kukuljan S, Daly RM (2010) Effects of lifetime loading history on cortical bone density and its distribution in middle-aged and older men. Bone 47(3):673–680. doi:10.1016/j.bone.2010.06.027
McNeil CJ, Raymer GH, Doherty TJ et al (2009) Geometry of a weight-bearing and non-weight-bearing bone in the legs of young, old, and very old men. Calcif Tissue Int 85(1):22–30. doi:10.1007/s00223-009-9261-7
Holzer G, von Skrbensky G, Holzer LA, Pichl W (2009) Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res 24(3):468–474. doi:10.1359/jbmr.081108
Koivumäki JEM, Thevenot J, Pulkkinen P et al (2012) Cortical bone finite element models in the estimation of experimentally measured failure loads in the proximal femur. Bone 51(4):737–740. doi:10.1016/j.bone.2012.06.026
Rantalainen T, Sievänen H, Linnamo V et al (2009) Bone rigidity to neuromuscular performance ratio in young and elderly men. Bone 45(5):956–963. doi:10.1016/j.bone.2009.07.014
Rantalainen T, Heinonen A, Komi PV, Linnamo V (2008) Neuromuscular performance and bone structural characteristics in young healthy men and women. Eur J Appl Physiol 102(2):215–222. doi:10.1007/s00421-007-0575-8
Korhonen MT, Heinonen A, Siekkinen J et al (2012) Bone density, structure and strength, and their determinants in aging sprint athletes. Med Sci Sports Exerc 44(12):2340–2349. doi:10.1249/MSS.0b013e318267c954
Rantalainen T, Hoffrén M, Linnamo V et al (2011) Three-month bilateral hopping intervention is ineffective in initiating bone biomarker response in healthy elderly men. Eur J Appl Physiol 111(9):2155–2162. doi:10.1007/s00421-011-1849-8
Ashe MC, Khan KM, Kontulainen SA et al (2006) Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 17(8):1241–1251. doi:10.1007/s00198-006-0110-5
Doube M, Kłosowski MM, Arganda-Carreras I et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47(6):1076–1079. doi:10.1016/j.bone.2010.08.023
Genant HK, Engelke K, Hanley DA et al (2010) Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 47(1):131–139. doi:10.1016/j.bone.2010.04.594
Nikander R, Kannus P, Dastidar P et al (2009) Targeted exercises against hip fragility. Osteoporos Int 20(8):1321–1328. doi:10.1007/s00198-008-0785-x
Weidauer LA, Binkley TL, Berry R, Specker BL (2013) Variation in cortical density within the cortical shell of individuals across a range in densities and ages. J Musculoskelet Neuronal Interact 13(1):89–96
Smock AJ, Hughes JM, Popp KL et al (2009) Bone volumetric density, geometry, and strength in female and male collegiate runners. Med Sci Sports Exerc 41(11):2026–2032. doi:10.1249/MSS.0b013e3181a7a5a2
Kontulainen S, Sievänen H, Kannus P et al (2003) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 18(2):352–359. doi:10.1359/jbmr.2003.18.2.352
Neil JMB, Schweitzer ME (2008) Humeral cortical and trabecular changes in the throwing athlete: a quantitative computed tomography study of male college baseball players. J Comput Assist Tomogr 32(3):492–496. doi:10.1097/RCT.0b013e31811ec72d
Ireland A, Maden-Wilkinson T, Mcphee J et al (2013) Upper limb muscle-bone asymmetries and bone adaptation in elite youth tennis players. Med Sci Sports Exerc 45:1749–1758. doi:10.1249/MSS.0b013e31828f882f
Jepsen KJ, Centi A, Duarte GF et al (2011) Biological constraints that limit compensation of a common skeletal trait variant lead to inequivalence of tibial function among healthy young adults. J Bone Miner Res 26(12):2872–2885. doi:10.1002/jbmr.497
Jepsen KJ, Evans R, Negus CH et al (2013) Variation in tibial functionality and fracture susceptibility among healthy, young adults arises from the acquisition of biologically distinct sets of traits. J Bone Miner Res 28(6):1290–1300. doi:10.1002/jbmr.1879
Giladi M, Milgrom C, Simkin A et al (1987) Stress fractures and tibial bone width. A risk factor. J Bone Joint Surg Br 69(2):326–329
Popp KL, Hughes JM, Smock AJ et al (2009) Bone geometry, strength, and muscle size in runners with a history of stress fracture. Med Sci Sports Exerc 41(12):2145–2150. doi:10.1249/MSS.0b013e3181a9e772
Roschger P, Paschalis EP, Fratzl P, Klaushofer K (2008) Bone mineralization density distribution in health and disease. Bone 42(3):456–466. doi:10.1016/j.bone.2007.10.021
Gan AWT, Puhaindran ME, Pho RWH (2013) The reconstruction of large bone defects in the upper limb. Injury 44(3):313–317. doi:10.1016/j.injury.2013.01.014
Atkins RM, Madhavan P, Sudhakar J, Whitwell D (1999) Ipsilateral vascularised fibular transport for massive defects of the tibia. J Bone Joint Surg Br 81(6):1035–1040
Ducher G, Daly RM, Hill B et al (2009) Relationship between indices of adiposity obtained by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in pre-pubertal children. Ann Hum Biol 36(6):705–716. doi:10.3109/03014460903055139
Burt LA, Naughton GA, Greene DA, Ducher G (2011) Skeletal differences at the ulna and radius between pre-pubertal non-elite female gymnasts and non-gymnasts. J Musculoskelet Neuronal Interact 11(3):227–233
Acknowledgments
The Academy of Finland (250683, 253198), Ministry of Education and Culture, KELA (Dnro 34/26/2011). Dr Rantalainen was supported by a Grant from Emil Aaltonen Foundation during the preparation of the mansucript.
Conflict of Interest
All authors state that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with the ethical standards of the ethical comittee of the University of Jyväskylä, Finland responsible on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rantalainen, T., Duckham, R.L., Suominen, H. et al. Tibial and Fibular Mid-Shaft Bone Traits in Young and Older Sprinters and Non-Athletic Men. Calcif Tissue Int 95, 132–140 (2014). https://doi.org/10.1007/s00223-014-9881-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9881-4