Abstract
Introduction
With reducing mortality in children with hematological malignancies, the survivors' quality of life regarding development of chronic neurological disturbances is important. We aimed to determine whether chemotherapy affects white matter (WM).
Methods
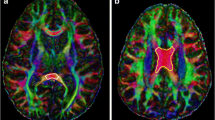
Using brain diffusion tensor imaging, we evaluated 17 patients (15 with acute lymphoblastic leukemia, 2 with non-Hodgkin's lymphoma; 9 male, 8 female; age, 1.6–13 years) before and after chemotherapy. We measured the quantitative values of fractional anisotropy (FA) and apparent diffusion coefficient (ADC) at the regions of interest (ROIs) such as periventricular WM, corona radiata, posterior limb of the internal capsule, and corpus callosum. We assessed sensorimotor and callosal tracts by tractography.
Results
Reduction in FA and increase in ADC were significant at the ROIs of the left and right anterior periventricular WM and corona radiata and at the tract passing through the genu. A significant reduction in FA with a nonsignificant increase in ADC was seen at the ROI of the genu and at the tracts passing through the body and isthmus.
Conclusion
Chemotherapy in children with hematological malignancies predominantly affects the frontal WM. This finding might indicate a negative effect of chemotherapy on neurological development in children with hematological malignancies.

Similar content being viewed by others
References
Pui C-H, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371(9617):1030–1043. doi:10.1016/s0140-6736(08)60457-2
Gross TG, Termuhlen AM (2007) Pediatric non-Hodgkin's lymphoma. Curr Oncol Rep 9(6):459–465
Mori S, Oishi K, Faria AV (2009) White matter atlases based on diffusion tensor imaging. Curr Opin Neurol 22(4):362–369. doi:10.1097/WCO.0b013e32832d954b
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66(1):259–267. doi:10.1016/s0006-3495(94)80775-1
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230(1):77–87. doi:10.1148/radiol.2301021640
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15(7–8):435–455. doi:10.1002/nbm.782
Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S (2006) Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. NeuroImage 29(2):493–504. doi:10.1016/j.neuroimage.2005.08.017
Wieshmann UC, Clark CA, Symms MR, Franconi F, Barker GJ, Shorvon SD (1999) Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magn Reson Imaging 17(9):1269–1274
McIntosh S, Klatskin EH, O'Brien RT, Aspnes GT, Kammerer BL, Snead C, Kalavsky SM, Pearson HA (1976) Chronic neurologic disturbance in childhood leukemia. Cancer 37(2):853–857
Langer T, Martus P, Ottensmeier H, Hertzberg H, Beck JD, Meier W (2002) CNS late-effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol 38(5):320–328. doi:10.1002/mpo.10055
Reddick WE, Shan ZY, Glass JO, Helton S, Xiong X, Wu S, Bonner MJ, Howard SC, Christensen R, Khan RB, Pui CH, Mulhern RK (2006) Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer 106(4):941–949. doi:10.1002/cncr.21679
Carey ME, Haut MW, Reminger SL, Hutter JJ, Theilmann R, Kaemingk KL (2008) Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. AJNR Am J Neuroradiol 29(4):792–797. doi:10.3174/ajnr.A0904
Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi GC, McAlonan G, Cao G, Chan GC (2006) White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol 24(6):884–890. doi:10.1200/JCO.2005.02.4505
Murakami A, Morimoto M, Yamada K, Kizu O, Nishimura A, Nishimura T, Sugimoto T (2008) Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics 122(3):500–506. doi:10.1542/peds.2007-2816
Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S (2005) DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. NeuroImage 26(1):195–205. doi:10.1016/j.neuroimage.2005.01.019
Hasegawa T, Yamada K, Morimoto M, Morioka S, Tozawa T, Isoda K, Murakami A, Chiyonobu T, Tokuda S, Nishimura A, Nishimura T, Hosoi H (2011) Development of corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr Res 69(3):249–254. doi:10.1203/PDR.0b013e3182084e54
Khong PL, Kwong DL, Chan GC, Sham JS, Chan FL, Ooi GC (2003) Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. AJNR Am J Neuroradiol 24(4):734–740
Reddick WE, Glass JO, Johnson DP, Laningham FH, Pui CH (2009) Voxel-based analysis of T2 hyperintensities in white matter during treatment of childhood leukemia. AJNR Am J Neuroradiol 30(10):1947–1954. doi:10.3174/ajnr.A1733
Kinney HC, Brody BA, Kloman AS, Gilles FH (1988) Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 47(3):217–234
Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D (1988) Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166(1 Pt 1):173–180
Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M (1999) Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport 10(13):2817–2821
McGraw P, Liang L, Provenzale JM (2002) Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol 179(6):1515–1522
Barkovich AJ (2005) Magnetic resonance techniques in the assessment of myelin and myelination. J Inherit Metab Dis 28(3):311–343. doi:10.1007/s10545-005-5952-z
Rodriguez M (2003) A function of myelin is to protect axons from subsequent injury: implications for deficits in multiple sclerosis. Brain 126(Pt 4):751–752
Edelstein K, D'Agostino N, Bernstein LJ, Nathan PC, Greenberg ML, Hodgson DC, Millar BA, Laperriere N, Spiegler BJ (2011) Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 33(6):450–458. doi:10.1097/MPH.0b013e31820d86f2
Acknowledgments
We thank Professor Ikumitsu Nagasaki, Department of Mathematics, Kyoto Prefectural University of Medicine, for his advice about statistical analyses.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morioka, S., Morimoto, M., Yamada, K. et al. Effects of chemotherapy on the brain in childhood: diffusion tensor imaging of subtle white matter damage. Neuroradiology 55, 1251–1257 (2013). https://doi.org/10.1007/s00234-013-1245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1245-7