Abstract
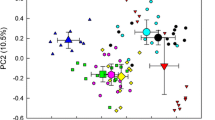
It is known that the microbial community of the rhizosphere is not only influenced by factors such as root exudates, phenology, and nutrient uptake but also by the plant species. However, studies of bacterial communities associated with tropical rainforest tree root surfaces, or rhizoplane, are lacking. Here, we analyzed the bacterial community of root surfaces of four species of native trees, Agathis borneensis, Dipterocarpus kerrii, Dyera costulata, and Gnetum gnemon, and nearby bulk soils, in a rainforest arboretum in Malaysia, using 454 pyrosequencing of the 16S rRNA gene. The rhizoplane bacterial communities for each of the four tree species sampled clustered separately from one another on an ordination, suggesting that these assemblages are linked to chemical and biological characteristics of the host or possibly to the mycorrhizal fungi present. Bacterial communities of the rhizoplane had various similarities to surrounding bulk soils. Acidobacteria, Alphaproteobacteria, and Betaproteobacteria were dominant in rhizoplane communities and in bulk soils from the same depth (0–10 cm). In contrast, the relative abundance of certain bacterial lineages on the rhizoplane was different from that in bulk soils: Bacteroidetes and Betaproteobacteria, which are known as copiotrophs, were much more abundant in the rhizoplane in comparison to bulk soil. At the genus level, Burkholderia, Acidobacterium, Dyella, and Edaphobacter were more abundant in the rhizoplane. Burkholderia, which are known as both pathogens and mutualists of plants, were especially abundant on the rhizoplane of all tree species sampled. The Burkholderia species present included known mutualists of tropical crops and also known N fixers. The host-specific character of tropical tree rhizoplane bacterial communities may have implications for understanding nutrient cycling, recruitment, and structuring of tree species diversity in tropical forests. Such understanding may prove to be useful in both tropical forestry and conservation.



Similar content being viewed by others
References
Brodie E, Edwards S, Clipson N (2002) Bacterial community dynamics across a floristic gradient in a temperate upland grassland ecosystem. Microbial Ecol 44:260–270
Fang CW, Radosevich M, Fuhrmann JJ (2001) Characterization of rhizosphere microbial community structure in five similar grass species using FAME and BIOLOG analyses. Soil Biol Biochem 33:679–682
Broughton LC, Gross KL (2000) Patterns of diversity in plant and soil microbial communities along a productivity gradient in a Michigan old-field. Oecologia 125:420–427
Kowalchuk GA, Buma DS, de Boer W, Klinkhamer PG, van Veen JA (2002) Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie van Leeuwenhoek 81:509–520
McCaig AE, Glover LA, Prosser JI (2001) Numerical analysis of grassland bacterial community structure under different land management regimens by using 16S ribosomal DNA sequence data and denaturing gradient gel electrophoresis banding patterns. Appl Environ Microbiol 67:4554–4559
Grayston SJ, Wang SQ, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microb 67:4742–4751
Wieland G, Neumann R, Backhaus H (2001) Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol 67:5849–5854
Dunfield KE, Germida JJ (2003) Seasonal changes in the rhizosphere microbial communities associated with field-grown genetically modified canola (Brassica napus). Appl Environ Microbiol 69:7310–7318
van Elsas JD, Speksnijder AJ, van Overbeek LS (2008) A procedure for the metagenomics exploration of disease-suppressive soils. J Microbiol Methods 75:515–522
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33:1437–1445
Buckley DH, Schmidt TM (2003) Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ Microbiol 5:441–452
Girvan MS, Bullimore J, Pretty JN, Osborn AM, Ball AS (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol 69:1800–1809
Johnson MJ, Lee KY, Scow KM (2003) DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 114:279–303
DiCello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63:4485–4493
Uroz S, Calvaruso C, Turpaul MP, Pierrat JC, Mustin C, Frey-Klett P (2007) Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl Environ Microbiol 73:3019–3027
Cocking EC (2003) Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 252:169–175
Nieto KF, Frankenberger WT (1989) Biosynthesis of cytokinins in soil. Soil Sci Soc Am J 53:735–740
Hamdan H, Weller DM, Thomashow LS (1991) Relative importance of fluorescent siderophores and other factors in biological-control of Gaeumannomyces graminis var tritici by Pseudomonas fluorescens 2-79 and M4-80r. Appl Environ Microbiol 57:3270–3277
Berg G, Zachow C, Lottmann J, Gotz M, Costa R, Smalla K (2005) Impact of plant species and site on rhizosphere-associated fungi antagonistic to Verticillium dahliae Kleb. Appl Environ Microbiol 71:4203–4213
Marilley L, Aragno M (1999) Phylogenetic diversity of bacterial communities differing in degree of proximity of Lolium perenne and Trifolium repens roots. Appl Soil Ecol 13:127–136
Marilley L, Vogt G, Blanc M, Aragno M (1998) Bacterial diversity in the bulk soil and rhizosphere fractions of Lolium perenne and Trifolium repens as revealed by PCR restriction analysis of 16S rDNA. Plant Soil 198:219–224
Acosta-Martinez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
StreitwolfEngel R, Boller T, Wiemken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J Ecol 85:181–191
Newsham KK, Fitter AH, Watkinson AR (1995) Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J Ecol 83:991–1000
Brundrett M (1991) Mycorrhizas in Natural Ecosystems. Adv Ecol Res 21:171–313
Miethling R, Wieland G, Backhaus H, Tebbe CC (2000) Variation of microbial rhizosphere communities in response to crop species, soil origin, and inoculation with Sinorhizobium meliloti L33. Microbial Ecol 40:43–56
McCaig AE, Glover LA, Prosser JI (1999) Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Appl Environ Microbiol 65:1721–1730
Nunan N, Daniell TJ, Singh BK, Papert A, McNicol JW, Prosser JI (2005) Links between plant and rhizoplane bacterial communities in grassland soils, characterized using molecular techniques. Appl Environ Microbiol 71:6784–6792
Teixeira LCRS, Peixoto RS, Cury JC, Sul WJ, Pellizari VH, Tiedje J, Rosado AS (2010) Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay, maritime Antarctica. ISME J 4:989–1001
Buyer JS, Roberts DP, Russek-Cohen E (1999) Microbial community structure and function in the spermosphere as affected by soil and seed type. Can J Microbiol 45:138–144
Dalmastri C, Chiarini L, Cantale C, Bevivino A, Tabacchioni S (1999) Soil type and maize cultivar affect the genetic diversity of maize root-associated Burkholderia cepacia populations. Microbial Ecol 38:273–284
Day JM, Neves MCP, Döbereiner J (1974) Nitrogenase activity on the roots of tropical forage grasses. Soil Biol Biochem 7:107–112
Döbereiner J, Day JM, Dart PJ (1972) Nitrogenase activity in the rhizosphere of sugar cane and some other tropical grasses. Plant Soil 37:191–196
Gomes NCM, Heuer H, Schonfeld J, Costa R, Mendonca-Hagler L, Smalla K (2001) Bacterial diversity of the rhizosphere of maize (Zea mays) grown in tropical soil studied by temperature gradient gel electrophoresis. Plant Soil 232:167–180
Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J (2009) Metagenomic pyrosequencing and microbial identification. Clin Chem 55:856–866
Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA (2009) Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433
Chun J, Kim KY, Lee JH, Choi Y (2010) The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol 10:101
Unno T, Jang J, Han D, Kim JH, Sadowsky MJ, Kim OS, Chun J, Hur HG (2010) Use of barcoded pyrosequencing and shared OTUs to determine sources of fecal bacteria in watersheds. Environ Sci Technol 44:7777–7782
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorials. Primer-E Ltd, Plymouth
White JR, Nagarajan N, Pop M (2009) Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Compute Biol 5:e1000352
Suarez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonca-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol 63:249–266
Caballero-Mellado J, Martinez-Aguilar L, Paredes-Valdez G, Santos PE (2004) Burkholderia unamae sp. nov., an N2-fixing rhizospheric and endophytic species. Int J Syst Evol Microbiol 54:1165–1172
Reis VM, Estrada-de los Santos P, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, Mavingui P, Baldani VL, Schmid M, Baldani JI, Balandreau J, Hartmann A, Caballero-Mellado J (2004) Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int J Syst Evol Microbiol 54:2155–2162
Chen WM, de Faria SM, Chou JH, James EK, Elliott GN, Sprent JI, Bontemps C, Young JP, Vandamme P (2008) Burkholderia sabiae sp. nov., isolated from root nodules of Mimosa caesalpiniifolia. Int J Syst Evol Microbiol 58:2174–2179
Caballero-Mellado J, Onofre-Lemus J, Estrada-de Los Santos P, Martinez-Aguilar L (2007) The tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Appl Environ Microbiol 73:5308–5319
Kim M, Singh D, Lai-Hoe A, Go R, Abdul Rahim R, NA A, Chun J, Adams JM (2012) Distinctive phyllosphere bacterial communities in tropical trees. Microb Ecol 63:674–681
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291
Singh BK, Nunan N, Ridgway KP, McNicol J, Young JPW, Daniell TJ, Prosser JI, Millard P (2008) Relationship between assemblages of mycorrhizal fungi and bacteria on grass roots. Environ Microbiol 10:534–541
Wamberg C, Christensen S, Jakobsen I, Muller AK, Sorensen SJ (2003) The mycorrhizal fungus (Glomus intraradices) affects microbial activity in the rhizosphere of pea plants (Pisum sativum). Soil Biol Biochem 35:1349–1357
Olsson PA, Wallander H (1998) Interactions between ectomycorrhizal fungi and the bacterial community in soils amended with various primary minerals. FEMS Microbiol Ecol 27:195–205
de Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811
Dixona RK, Gargb VK, Raoc MV (1993) Inoculation of Leucaena and Prosopis seedlings with Glomus and Rhizobium species in saline soil: rhizosphere relations and seedling growth. Arid Soil Res Rehabil 7:133–144
Mangan SA, Schnitzer SA, Herre EA, Mack KM, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755
Timonen S, Hurek T (2006) Characterization of culturable bacterial populations associating with Pinus sylvestris–Suillus bovinus mycorrhizospheres. Can J Microbiol 52:769–778
Kielak A, Pijl AS, van Veen JA, Kowalchuk GA (2008) Differences in vegetation composition and plant species identity lead to only minor changes in soil-borne microbial communities in a former arable field. FEMS Microbiol Ecol 63:372–382
Gyaneshwar P, Hirsch AM, Moulin L, Chen WM, Elliott GN, Bontemps C, Estrada-de los Santos P, Gross E, DosReis FB, Sprent JI, Young JPW, James EK (2011) Legume-nodulating Betaproteobacteria: diversity, host range, and future prospects. Mol Plant Microbe In 24:1276–1288
Pagan JD, Child JJ, Scowcroft WR, Gibson AH (1975) Nitrogen-fixation by Rhizobium cultured on a defined medium. Nature 256:406–407
Kim M, Singh D, Lai-Hoe A, Go R, Abdul Rahim R, A NA, Chun J, Adams JM (2011) Distinctive phyllosphere bacterial communities in tropical trees. Microb Ecol
Chiu CH, Waddingdon M, Hsieh WS, Greenberg D, Schreckenberger PC, Carnahan AM (2000) Atypical Chryseobacterium meningosepticum and meningitis and sepsis in newborns and the immunocompromised, Taiwan. Emerg Infect Dis 6:481–486
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Yergeau E, Kang S, He Z, Zhou J, Kowalchuk GA (2007) Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J 1:163–179
Brejda JJ, Moser LE, Vogel KP (1998) Evaluation of switchgrass rhizosphere microflora for enhancing seedling yield and nutrient uptake. Agron J 90:753–758
Edwards DP, Fisher B, Boyd E (2010) Protecting degraded rainforests: enhancement of forest carbon stocks under REDD. Conserv Lett 3:313–316
Acknowledgements
J.M.A. was assisted by a Brain Gain Grant from the Government of Malaysia, administered through MOSTI.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 164 kb)
Rights and permissions
About this article
Cite this article
Oh, Y.M., Kim, M., Lee-Cruz, L. et al. Distinctive Bacterial Communities in the Rhizoplane of Four Tropical Tree Species. Microb Ecol 64, 1018–1027 (2012). https://doi.org/10.1007/s00248-012-0082-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0082-2