Abstract
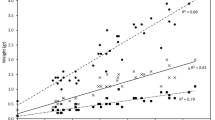
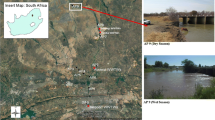
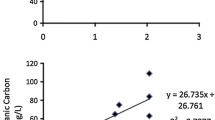
Faecal contamination poses health risks for the recreational users of urban estuaries. However, our understanding of the potential pathogenicity of faecal microbes in these environments is limited. To this end, a study was conducted to understand the spatial and seasonal distribution of Salmonella in water and sediments of the Yarra River estuary, Melbourne, Australia. Among 210 samples in total, culturable Salmonella were recovered from 27%, 17%, and 19% of water, bank, and bed sediment samples, respectively. The combined detection increased from 15% in winter to 32% in summer (p < 0.05) indicating seasonal variation as potential part of public health risk assessments. Further, pathogenic potential of the Salmonella isolates was characterised via the quantification of attachment and invasion capacity using human epithelial colorectal cell line Caco-2 on a subset of isolates (n = 62). While all of these isolates could attach and invade Caco-2 cells, 52% and 13% of these showed greater attachment and invasiveness, respectively, than the corresponding mean values for S. Typhimurium ATCC14028 control. Isolates from winter were on average more invasive (seven out of eight isolates with the highest invasiveness recovered from the colder sampling period) than the isolates from summer, and Salmonella collected during summer showed lower invasion (p < 0.05) compared with the control. Similar low invasion compared with the same control was observed for isolates recovered from bank sediment (p < 0.05). While the higher prevalence in summer may imply higher risks during these peak recreational periods, it is essential that this information is used in combination with quantitative microbial risk assessments to fully understand the health risks posed by Salmonella in microtidal estuaries.




Similar content being viewed by others
References
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Mallin MA, Williams KE, Esham EC, Lowe RP (2000) Effect of human development on bacteriological water quality in coastal watersheds. Ecol Appl 10:1047–1056
Henry R, Schang C, Chandrasena GI, Deletic A, Edmunds M, Jovanovic D, Kolotelo P, Schmidt J, Williamson R, McCarthy D (2015) Environmental monitoring of waterborne Campylobacter: evaluation of the Australian standard and a hybrid extraction-free MPN-PCR method. Front Microbiol 6:74
Ahmed W, Sawant S, Huygens F, Goonetilleke A, Gardner T (2009) Prevalence and occurrence of zoonotic bacterial pathogens in surface waters determined by quantitative PCR. Water Res 43:4918–4928
Catalao DLP, Joao M, Ferreiro VS, Fidalgo ML, Garcia Rosado ME, Borrego JJ (2000) Occurrence of Salmonella spp. in estuarine and coastal waters of Portugal. Antonie Van Leeuwenhoek 78:99–106
Cabral JPS (2010) Water microbiology. Bacterial pathogens and water. Int J Environ Res Public Health 7(10):3657–3703
EPA US (2012) Recreational water quality criteria. Health and Ecological Criteria Division, Office of Science and Technology, United States (U.S.) Environmental Protection Agency (EPA)
Odonkor ST, Ampofo JK (2013) Escherichia coli as an indicator of bacteriological quality of water: an overview. Microbiol Res 4(1):e2
Edberg SC, Rice EW, Karlin RJ, Allen MJ (2000) Escherichia coli: the best biological drinking water indicator for public health protection. Symp Ser Soc Appl Microbiol 88:106S–116S
Lemarchand K, Lebaron P (2003) Occurrence of Salmonella spp. and Cryptosporidium spp. in a French coastal watershed: relationship with fecal indicators. FEMS Microbiol Lett 218:203–209
Ferguson CM, Coote BG, Ashbolt NJ, Stevenson IM (1996) Relationships between indicators, pathogens and water quality in an estuarine system. Water Res 30:2045–2054
Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ (2000) Sources of Escherichia coli in a coastal subtropical environment. Appl Env Microbiol 66:230–237
Hoge CW, Watsky D, Peeler RN, Libonati JP, Israel E, Morris JG (1989) Epidemiology and spectrum of Vibrio infections in a Chesapeake Bay community. J Infect Dis 160:985–993
Lepesteur M, Pond D, McComb A, Ho G, Moore SA (2003) Fecal waste and the natural environment: water quality and epidemiological implications. ORBIT 2003 Org Recover Biol Treat Proc Fourth Int Conf ORBIT Assoc Biol Process Org Adv a Sustain Soc. Perth, Western Australia
NHMRC (2008) Guidelines for managing risks in recreational water. Australian Government
EPA US (2009) Fact Sheet: Final Third Drinking Water Contaminant Candidate List (CCL 3).
WHO (2013) Salmonella (non-typhoidal). Factsheet.
Gobius K, Fagan N, Moir C, Tyers P (2016) Salmonella: what is it, and how can you protect against it? CSIRO, Australia
Soller JA, Bartrand T, Ashbolt NJ, Ravenscroft J, Wade TJ (2010) Estimating the primary etiologic agents in recreational freshwaters impacted by human sources of fecal contamination. Water Res 44:4736–4747
Schoen ME, Ashbolt NJ (2010) Assessing pathogen risk to swimmers at non-sewage impacted recreational beaches. Environ Sci Technol 44:2286–2291
Westrell T, Schonning C, Stenstrom TA, Ashbolt NJ (2004) QMRA (quantitative microbial risk assessment) and HACCP (hazard analysis and critical control points) for management of pathogens in wastewater and sewage sludge treatment and reuse. Water Sci Technol 50:23–30
Efstratiou MA (2001) Managing coastal bathing water quality: the contribution of microbiology and epidemiology. Mar Pollut Bull 42:424–431
Pasmans F, Martel A, Boyen F, Vandekerchove D, Wybo I, Van Immerseel F, Heyndrickx M, Collard JM, Ducatelle R, Haesebrouck F (2005) Characterization of Salmonella isolates from captive lizards. Vet Microbiol 110:285–291
Tran TP, Ly TLK, Nguyen TT, Akiba M, Ogasawara N, Shinoda D, Okatani AT, Hayashidani H (2004) Prevalence of Salmonella spp. in pigs, chickens and ducks in the Mekong Delta, Vietnam. J Vet Med Sci 66:1011–1014
Kelterborn E (1967) Salmonella species1st edn. Springer Netherlands, Dordrecht
O’Keefe B, D’Arcy BJ, Davidson J, Barbarito B, Clelland B (2005) Urban diffuse sources of fecal indicators. Water Sci Technol 51(3-4):183–190
Daly E, Kolotelo P, Schang C, Osborne CA, Coleman R, Deletic A, McCarthy DT (2013) Escherichia coli concentrations and loads in an urbanised catchment: The Yarra River, Australia. J Hydrol 497:51–61
Vereen E, Lowrance RR, Cole DJ, Lipp EK (2007) Distribution and ecology of campylobacters in coastal plain streams (Georgia, United States of America). Appl Environ Microbiol 73:1395–1403
Hardisty J (2007) Estuaries: monitoring and modelling the physical system. Blackwell Publishing, Hoboken
Feng F, Goto D, Yan T (2010) Effects of autochthonous microbial community on the die-off of fecal indicators in tropical beach sand. FEMS Microbiol Ecol 74:214–225
Ahn JH (2012) Size distribution and settling velocities of suspended particles in a tidal embayment. Water Res 46:3219–3228
Thayer DW, Muller WS, Buchanan RL, Phillips JG (1987) Effect of NaCl, pH, temperature, and atmosphere on growth of Salmonella typhimurium in glucose-mineral salts medium. Appl Environ Microbiol 53:1311–1315
Perkins TL, Clements K, Baas JH, Jago CF, Jones DL, Malham SK, McDonald JE (2014) Sediment composition influences spatial variation in the abundance of human pathogen indicator bacteria within an estuarine environment. PLoS One 14;9(11):e112951
Chandran A, Mohamed Hatha AA (2005) Relative survival of Escherichia coli and Salmonella typhimurium in a tropical estuary. Water Res 39:1397–1403
Rhodes MW, Kator H (1988) Survival of Escherichia coli and Salmonella spp. in estuarine environments. Appl Environ Microbiol 54:2902–2907
Touron A, Berthe T, Pawlak B, Petit F (2005) Detection of Salmonella in environmental water and sediment by a nested-multiplex polymerase chain reaction assay. Res Microbiol 156:541–553
Polo F, Figueras MJ, Inza I, Sala J, Fleisher JM, Guarro J (1999) Prevalence of Salmonella serotypes in environmental waters and their relationships with indicator organisms. Antonie Van Leeuwenhoek 75:285–292
Lipp EK, Kurz R, Vincent R, Rodriguez-Palacios C, Farrah SR, Rose JB (2001) The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries 24:266–276
Ortega CI, Solo-Gabriele HM, Abdelzaher A, Wright M, Deng Y, Stark LM (2009) Correlations between microbial indicators, pathogens, and environmental factors in a subtropical estuary. Mar Pollut Bull 58(9):1374–1381
Martinez-Manzanares E, Moriñigo MA, Castro D, Balebona MC, Sanchez JM, Borrego JJ (1992) Influence of the fecal pollution of marine sediments on the microbial content of shellfish. Mar Pollut Bull 24:342–349
Desmarais TR, Solo-Gabriele HM, Palmer CJ (2002) Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl Env Microbiol 68:1165–1172
Siddiqee MH, Henry R, Coulthard R, Schang C, Williamson R, Coleman R, Rooney G, Deletic A, McCarthy D (2018) Salmonella enterica serovar Typhimurium and Escherichia coli survival in estuarine bank sediments. Int J Environ Res Public Health 15:2597
Bordalo AA, Onrassami R, Dechsakulwatana C (2002) Survival of fecal indicator bacteria in tropical estuarine waters (Bangpakong River, Thailand). J Appl Microbiol 93:864–871
Liu L, Chen L, Floehr T, Xiao H, Bluhm K, Hollert H, Wu L (2015) Assessment of the mutagenicity of sediments from Yangtze River estuary using Salmonella Typhimurium microsome assay. PLoS One 10(11):e0143522
Monfort P, Baleux B (1994) Effects of environmental factors present in the St. Lawrence estuary (Quebec, Canada) on experimental survival of Salmonella salamae as determined by flow cytometry. Can J Microbiol 40:712–719
Zaafrane S, Maatouk K (2012) Role of the rpoS gene in the survival of Salmonella enterica serovar Typhimurium in the marine environment. Water Qual Res J Canada 47:186–196
Yang Y, Khoo WJ, Zheng Q, Chung HJ, Yuk HG (2014) Growth temperature alters Salmonella Enteritidis heat/acid resistance, membrane lipid composition and stress/virulence related gene expression. Int J Food Microbiol 172:102–109
Parvathi A, Vijayan J, Murali G, Chandran P (2011) Comparative virulence genotyping and antimicrobial susceptibility profiling of environmental and clinical Salmonella enterica from Cochin, India. Curr Microbiol 62:21–26
Ginocchio CC, Rahn K, Clarke RC, Galán JE (1997) Naturally occurring deletions in the centisome 63 pathogenicity island of environmental isolates of Salmonella spp. Infect Immun 65:1267–1272
Marin PE, Suárez EP, Jiménez VO, Velilla SM (2006) Presence of the invasive gene invA in Salmonella spp. strains isolated from food in several cities of the Colombian Caribbean area. Rev Cuba Salud Publica:32
Sanchez-Jimenez MM, Cardona-Castro N, Canu N, Uzzau S, Rubino S (2010) Distribution of pathogenicity islands among Colombian isolates of Salmonella. J Infect Dev Ctries. 4:555–559
Lesne J, Berthet S, Binard S, Rouxel A, Humbert F (2000) Changes in culturability and virulence of Salmonella typhimurium during long-term starvation under desiccating conditions. Int J Food Microbiol 60:195–203
Soller JA, Schoen ME, Bartrand T, Ravenscroft JE, Ashbolt NJ (2010) Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of fecal contamination. Water Res 44:4674–4691
Moal VLL, Servina AL (2013) Pathogenesis of human enterovirulent bacteria: Lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol Mol Biol Rev 77:380–439
McCormick BA (2003) The use of transepithelial models to examine host-pathogen interactions. Curr Opin Microbiol 6:77–81
Gahring LC, Heffron F, Finlay BB, Falkow S (1990) Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun 58:443–448
Martins M, Mccusker MP, Mccabe E (2014) Evidence of metabolic switching and implications for food safety from the phenome(s) of Salmonella enterica Serovar Typhimurium DT104 cultured at selected points across the pork production food chain. Appl Environ Microbiol 79(18):5437–5449
McWhorter AR, Chousalkar KK (2015) Comparative phenotypic and genotypic virulence of Salmonella strains isolated from Australian layer farms. Front Microbiol 6:12
McWhorter AR, Davos D, Chousalkar KK (2015) Pathogenicity of Salmonella strains isolated from egg shells and the layer farm environment in Australia. Appl Environ Microbiol 81:405–414
Bolton AJ, Osborne MP, Stephen J (2000) Comparative study of the invasiveness of Salmonella serotypes Typhimurium, Choleraesuis and Dublin for Caco-2 cells, HEp-2 cells and rabbit ileal epithelia. J Med Microbiol. 49:503–511
Jovanovic D, Henry R, Coleman R, Deletic A, McCarthy DT (2015) Integrated conceptual modelling of fecal contamination in an urban estuary catchment. Water Sci Technol 72(9):1472–1480
Cabelli VJ (1989) Swimming-associated illness and recreational water quality criteria. Water Sci Technol 21:13–21
Schang C, Henry R, Kolotelo PA, Prosser T, Crosbie N, Grant T, Cottam D, O’Brien P, Coutts S, Deletic A, McCarthy DT (2016) Evaluation of techniques for measuring microbial hazards in bathing waters : a comparative study. PLoS One 11(5):e0155848
Department of Sustainability And Environment (2012) A cleaner Yarra River and Port Phillip Bay — a plan of action. Melbourne, Australia
Jovanovic D, Barnes MP, Teakle IAL, Bruce L, McCarthy DT (2015) 3D Hydrodynamics and vertical mixing in a stratified estuary, p. 1331–1337. IMACS World Congress and MODSIM International Congress on Modelling and Simulation
Gorski L, Parker CT, Liang A, Cooley MB, Jay-Russell MT, Gordus AG, Atwill ER, Mandrell RE (2011) Prevalence, distribution, and diversity of Salmonella enterica in a major produce region of California. Appl Environ Microbiol 77:2734–2748
Desin TS, Lam PK, Koch B, Mickael C, Berberov E, Wisner AL, Townsend HG, Potter AA, Koster W (2009) Salmonella enterica serovar Enteritidis pathogenicity island 1 is not essential for but facilitates rapid systemic spread in chickens. Infect Immun 77:2866–2875
Elhadad D, Desai P, Grassl GA, McClelland M, Rahav G, Gal-Mor O (2016) Differences in host cell invasion and Salmonella pathogenicity island 1 expression between Salmonella enterica serovar Paratyphi A and nontyphoidal S. Typhimurium. Infect Immun 84:1150–1165
Bornmann L, Leydesdorff L, Mutz R (2013) The use of percentiles and percentile rank classes in the analysis of bibliometric data: opportunities and limits. J Informetr 7:158–165
Kragten J (1994) Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst 119:2161–2165
Kaper JB, Sayler GS, Baldini MM, Colwell RR (1977) Ambient temperature primary nonselective enrichment for isolation of Salmonella spp. from an estuarine environment. Appl Environ Microbiol 33:829–835
Sayler GS, Nelson Jr JD, Justice A, Colwell RR (1976) Incidence of Salmonella spp., Clostridium botulinum, and Vibrio parahaemolyticus in an estuary. Appl Environ Microbiol 31:723–730
Knight IT, DiRuggiero J, Colwell RR (1991) Direct detection of enteropathogenic bacteria in estuarine water using nucleic acid probes. Water Sci Technol 24:261–266
Papapetropoulou M, Moschopoulos H (1996) Detection of Salmonella spp. in estuarine waters by using both the conventional culture and a probe technique. Water Air Soil Pollut 89:159–165
Hsu CY, Hsu BM, Chang TY, Hsu TK, Shen SM, Chiu YC, Wang HJ, Ji WT, Fan CW, Chen JL (2014) Evaluation of immunomagnetic separation for the detection of Salmonella in surface waters by polymerase chain reaction. Int J Environ Res Public Health 11:9811–9821
Britton E, Hales S, Venugopal K, Baker MG (2010) Positive association between ambient temperature and salmonellosis notifications in New Zealand, 1965-2006. Aust N Z J Public Heal 34:126–129
Stephen DM, Barnett AG (2016) Effect of temperature and precipitation on salmonellosis cases in South-East Queensland, Australia: an observational study. BMJ Open 25;6(2):e010204
Haley BJ, Cole DJ, Lipp EK (2009) Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Appl Environ Microbiol 75:1248–1255
Simental L, Martinez-Urtaza J (2008) Climate patterns governing the presence and permanence of salmonellae in coastal areas of Bahia de Todos Santos, Mexico. Appl Env Microbiol 74:5918–5924
Gupte AR, De Rezende CL, Joseph SW (2003) Induction and resuscitation of viable but nonculturable Salmonella enterica serovar Typhimurium DT104. Appl Env Microbiol 69:6669–6675
Jovanovic D, Coleman R, Deletic A, McCarthy DT (2017) Tidal fluctuations influence E. coli concentrations in urban estuaries. Mar Pollut Bull 119:226–230
Fàbrega A, Vila J (2013) Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26:308–341
Dostal A, Gagnon M, Chassard C, Zimmermann MB, O’Mahony L, Lacroix C (2014) Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS One 9:e93549
Lehto EM, Salminen SJ (1997) Cell cultures by Salmonella typhimurium adhesion to Caco-2 Lactobacillus strain GG spent culture supernate : only a pH effect? FEMS Immunol Med Microbiol 18(2):125–132
Mccormick BA, Colgan SP, Miller I (1993) Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol 123:895–907
Rouch DA, Policy C, Group S, Deighton M (2011) Salmonella prevalence during sewage sludge treatment. AWA OzWater ’11 Conference. Adelaide, Australia
Theitler DJ, Nasser A, Gerchman Y, Kribus A, Mamane H (2012) Synergistic effect of heat and solar UV on DNA damage and water disinfection of E. coli and bacteriophage MS2. J Water Health 10:605–618
Rastogi RP, Richa KA, Tyagi MB, Sinha RP (2010) Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids 2010:592980
Ssemakalu CC, Woulter LR, Pillay M (2013) Impact of solar irradiation on cholera toxin secretion by different strains of Vibrio cholerae. S Afr J Sci 109:1–6
Mennigmann HD (1975) Inactivating and mutagenic effects of long-wave UV-light in bacteria with various mechanism of repair. Strahlentherapie 150:194
Runkel S, Wells HC, Rowley G (2013) Living with stress. a lesson from the enteric pathogen Salmonella enterica. Adv Appl Microbiol 83:87–144
Bruscolini F, Barbieri F, Battistelli M, Betti M, Dominici S, Manti A, Boi P, Marinelli F, Papa S, Pianetti A (2014) A multi-approach study of influence of growth temperature and nutrient deprivation in a strain of Aeromonas hydrophila. Int J Food Microbiol 188:1–10
Wanjugi P, Harwood VJ (2013) The influence of predation and competition on the survival of commensal and pathogenic fecal bacteria in aquatic habitats. Environ Microbiol 15:517–526
Xie W, Zhang C, Zhou X, Wang P (2014) Salinity-dominated change in community structure and ecological function of Archaea from the lower Pearl River to coastal South China Sea. Appl Microbiol Biotechnol 98:7971–7982
Almeida F, Medeiros MIC, Rodrigues DP, Falcão JP (2015) Genotypic diversity, pathogenic potential and the resistance profile of Salmonella Typhimurium strains isolated from humans and food from 1983 to 2013 in Brazil. J Med Microbiol 64:1395–1407
Grange ZL, Biggs PJ, Rose SP, Gartrell BD, Nelson NJ, French NP (2017) Genomic epidemiology and management of Salmonella in island ecosystems used for Takahe conservation. Microb Ecol 74:735–744
Jacobsen A, Hendriksen RS, Aaresturp FM, Ussery DW, Friis C (2011) The Salmonella enterica pan-genome. Microb Ecol 62:487–504
Acknowledgements
We also acknowledge Prof. Anton Peleg and Prof. Charles Mackay of Monash University for providing the opportunity to use the cell-culture facility and providing the Caco-2 cells, respectively. We are also grateful to Mr. Scott Coutts from Micromon, Monash University, for his magnetic separator that was used in this experiment.
Funding
We gratefully acknowledge that this study was supported by funding contributions from Melbourne Water and the Australian Research Council (LP120100718).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqee, M.H., Henry, R., Deletic, A. et al. Salmonella from a Microtidal Estuary Are Capable of Invading Human Intestinal Cell Lines. Microb Ecol 79, 259–270 (2020). https://doi.org/10.1007/s00248-019-01419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01419-2