Abstract
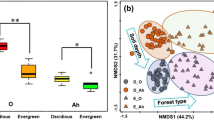
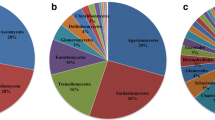
Abiotic and biotic drivers of co-occurring fungal functional guilds across regional-scale environmental gradients remain poorly understood. We characterized fungal communities using Illumina sequencing from soil cores collected across three Neotropical rainforests in Panama that vary in soil properties and plant community composition. We classified each fungal OTU into different functional guilds, namely plant pathogens, saprotrophs, arbuscular mycorrhizal (AM), or ectomycorrhizal (ECM). We measured soil properties and nutrients within each core and determined the tree community composition and richness around each sampling core. Canonical correspondence analyses showed that soil pH and moisture were shared potential drivers of fungal communities for all guilds. However, partial the Mantel tests showed different strength of responses of fungal guilds to composition of trees and soils. Plant pathogens and saprotrophs were more strongly correlated with soil properties than with tree composition; ECM fungi showed a stronger correlation with tree composition than with soil properties; and AM fungi were correlated with soil properties, but not with trees. In conclusion, we show that co-occurring fungal guilds respond differently to abiotic and biotic environmental factors, depending on their ecological function. This highlights the joint role that abiotic and biotic factors play in determining composition of fungal communities, including those associated with plant hosts.


Similar content being viewed by others
References
A’Bear AD, Jones TH, Kandeler E, Boddy L (2014) Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol Biochem 70:151–158. https://doi.org/10.1016/j.soilbio.2013.12.017
Abarenkov K, Henrik Nilsson R, Larsson K-H et al (2010) The UNITE database for molecular identification of fungi – recent updates and future perspectives. New Phytol 186:281–285. https://doi.org/10.1111/j.1469-8137.2009.03160.x
Albornoz FE, Lambers H, Turner BL et al (2016a) Shifts in symbiotic associations in plants capable of forming multiple root symbioses across a long-term soil chronosequence. Ecol Evol 6:2368–2377. https://doi.org/10.1002/ece3.2000
Albornoz FE, Teste FP, Lambers H et al (2016b) Changes in ectomycorrhizal fungal community composition and declining diversity along a 2-million-year soil chronosequence. Mol Ecol 25:4919–4929. https://doi.org/10.1111/mec.13778
Augspurger CK (1983) Seed dispersal of the tropical tree, Platypodium elegans, and the escape of its seedlings from fungal pathogens. J Ecol 71:759–771. https://doi.org/10.2307/2259591
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545. https://doi.org/10.1038/nature12901
Bagchi R, Swinfield T, Gallery RE et al (2010) Testing the Janzen-Connell mechanism: pathogens cause overcompensating density dependence in a tropical tree. Ecol Lett 13:1262–1269. https://doi.org/10.1111/j.1461-0248.2010.01520.x
Bagchi R, Gallery RE, Gripenberg S et al (2014) Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506:85–88. https://doi.org/10.1038/nature12911
Bahram M, Põlme S, Kõljalg U et al (2012) Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol 193:465–473. https://doi.org/10.1111/j.1469-8137.2011.03927.x
Baldrian P (2009) Ectomycorrhizal fungi and their enzymes in soils: is there enough evidence for their role as facultative soil saprotrophs? Oecologia 161:657–660. https://doi.org/10.1007/s00442-009-1433-7
Barberán A, McGuire KL, Wolf JA et al (2015) Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol Lett 18:1397–1405. https://doi.org/10.1111/ele.12536
Bell T, Freckleton RP, Lewis OT (2006) Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol Lett 9:569–574. https://doi.org/10.1111/j.1461-0248.2006.00905.x
Brown JKM, Tellier A (2011) Plant-parasite coevolution: bridging the gap between genetics and ecology. Annu Rev Phytopathol 49:345–367. https://doi.org/10.1146/annurev-phyto-072910-095301
Buée M, Maurice J-P, Zeller B et al (2011) Influence of tree species on richness and diversity of epigeous fungal communities in a French temperate forest stand. Fungal Ecol 4:22–31. https://doi.org/10.1016/j.funeco.2010.07.003
Campos-Soriano L, García-Martínez J, Segundo BS (2012) The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol Plant Pathol 13:579–592. https://doi.org/10.1111/j.1364-3703.2011.00773.x
Carson WP, Anderson JT, Leigh E, Schnitzer SA (2008) Challenges associated with testing and falsifying the Janzen-Connell hypothesis: a review and critique. Tropical Forest Community Ecology 210–241
Chen Y-L, Xu T-L, Veresoglou SD et al (2017) Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol Biochem 110:12–21. https://doi.org/10.1016/j.soilbio.2017.02.015
Clark DA, Clark DB (1984) Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen-Connell model. Am Nat 124:769–788
Clemmensen KE, Bahr A, Ovaskainen O et al (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618. https://doi.org/10.1126/science.1231923
Condit R, Engelbrecht BMJ, Pino D et al (2013a) Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc Natl Acad Sci 110:5064–5068. https://doi.org/10.1073/pnas.1218042110
Condit R, Pẽrez R, Aguilar S, Lao S (2013b) Data from tree censuses and inventories in Panama; https://doi.org/10.5479/data.stri.2016.0622
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. Dyn Popul 298:312
Core Team R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Corrales A, Turner BL, Tedersoo L et al (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol 27:14–23. https://doi.org/10.1016/j.funeco.2017.02.004
Coughlan AP, Dalpé Y, Lapointe L, Piché Y (2000) Soil pH-induced changes in root colonization, diversity, and reproduction of symbiotic arbuscular mycorrhizal fungi from healthy and declining maple forests. Can J For Res 30:1543–1554. https://doi.org/10.1139/x00-090
de Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811. https://doi.org/10.1016/j.femsre.2004.11.005
Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat Rev Genet 11:539–548. https://doi.org/10.1038/nrg2812
Duchesne LC, Peterson RL, Ellis BE (1988) Pine root exudate stimulates the synthesis of antifungal compounds by the ectomycorrhizal fungus Paxillus involutus. New Phytol 108:471–476. https://doi.org/10.1111/j.1469-8137.1988.tb04188.x
Edgar RC (2013) UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Engelbrecht BMJ, Comita LS, Condit R et al (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447:80–82. https://doi.org/10.1038/nature05747
Essene AL, Shek KL, Lewis JD, Peay KG, McGuire KL (2017) Soil type has a stronger role than Dipterocarp host species in shaping the ectomycorrhizal fungal community in a Bornean lowland tropical rain forest. Frontiers in plant science 8:1828
Fang X, You MP, Barbetti MJ (2012) Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur J Plant Pathol 134:619–629. https://doi.org/10.1007/s10658-012-0042-1
Fernandez CW, Kennedy PG (2016) Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209:1382–1394. https://doi.org/10.1111/nph.13648
Gadgil RL, Gadgil PD (1971) Mycorrhiza and litter decomposition. Nature 233:133. https://doi.org/10.1038/233133a0
Gadgil PD, Gadgil RL (1975) Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. N Z J For Sci 5:33–41
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Gómez-Aparicio L, Ibáñez B, Serrano MS et al (2012) Spatial patterns of soil pathogens in declining Mediterranean forests: implications for tree species regeneration. New Phytol 194:1014–1024. https://doi.org/10.1111/j.1469-8137.2012.04108.x
Haas SE, Hall Cushman J, Dillon WW et al (2016) Effects of individual, community, and landscape drivers on the dynamics of a wildland forest epidemic. Ecology 97:649–660. https://doi.org/10.1890/15-0767.1
Hantsch L, Braun U, Scherer-Lorenzen M, Bruelheide H (2013) Species richness and species identity effects on occurrence of foliar fungal pathogens in a tree diversity experiment. Ecosphere 4:1–12. https://doi.org/10.1890/ES13-00103.1
Hantsch L, Bien S, Radatz S et al (2014) Tree diversity and the role of non-host neighbour tree species in reducing fungal pathogen infestation. J Ecol 102:1673–1687. https://doi.org/10.1111/1365-2745.12317
Harvell CD, Mitchell CE, Ward JR et al (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. https://doi.org/10.1126/science.1063699
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218. https://doi.org/10.1146/annurev.ecolsys.36.112904.151932
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hempel S, Renker C, Buscot F (2007) Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ Microbiol 9:1930–1938. https://doi.org/10.1111/j.1462-2920.2007.01309.x
Herre EA, Kyllo D, Mangan S, et al (2005) An overview of arbuscular mycorrhizal fungi composition, distribution, and host effects from a tropical moist forest. In: Burslem D, Pinard M, Hartley S (eds) Biotic interactions in the tropics: Their role in the maintenance of species diversity. Cambridge University Press, Cambridge, UK, pp 204–225
Hiiesalu I, Bahram M, Tedersoo L (2017) Plant species richness and productivity determine the diversity of soil fungal guilds in temperate coniferous forest and bog habitats. Mol Ecol 26:4846–4858. https://doi.org/10.1111/mec.14246
Hobbie SE, Gough L (2004) Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140:113–124. https://doi.org/10.1007/s00442-004-1556-9
Hovmøller MS, Yahyaoui AH, Milus EA, Justesen AF (2008) Rapid global spread of two aggressive strains of a wheat rust fungus. Mol Ecol 17:3818–3826. https://doi.org/10.1111/j.1365-294X.2008.03886.x
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528. https://doi.org/10.1086/282687
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Keesing F, Holt RD, Ostfeld RS (2006) Effects of species diversity on disease risk. Ecol Lett 9:485–498. https://doi.org/10.1111/j.1461-0248.2006.00885.x
Kivlin SN, Hawkes CV (2016) Tree species, spatial heterogeneity, and seasonality drive soil fungal abundance, richness, and composition in Neotropical rainforests. Environ Microbiol 18:4662–4673
Koide RT, Wu T (2003) Ectomycorrhizas and retarded decomposition in a Pinus resinosa plantation. New Phytol 158:401–407. https://doi.org/10.1046/j.1469-8137.2003.00732.x
Koide RT, Fernandez C, Malcolm G (2014) Determining place and process: functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol 201:433–439. https://doi.org/10.1111/nph.12538
Kõljalg U, Larsson K-H, Abarenkov K et al (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068. https://doi.org/10.1111/j.1469-8137.2005.01376.x
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097. https://doi.org/10.1890/08-1555.1
Krüger M, Teste FP, Laliberté E et al (2015) The rise and fall of arbuscular mycorrhizal fungal diversity during ecosystem retrogression. Mol Ecol 24:4912–4930. https://doi.org/10.1111/mec.13363
Kubartová A, Ranger J, Berthelin J, Beguiristain T (2009) Diversity and decomposing ability of saprophytic fungi from temperate forest litter. Microb Ecol 58:98–107. https://doi.org/10.1007/s00248-008-9458-8
Lee C-S, Lee Y-J, Jeun Y-C (2005) Observations of infection structures on the leaves of cucumber plants pre-treated with arbuscular mycorrhiza Glomus intraradices after challenge inoculation with Colletotrichum orbiculare. Plant Pathol J 21:237–243. https://doi.org/10.5423/PPJ.2005.21.3.237
Legendre P, Legendre L (2012) Chapter 11 - Canonical analysis. In: Legendre P, Legendre L (eds) Developments in Environmental Modelling. Elsevier, pp 625–710
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi – potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447. https://doi.org/10.1111/nph.13201
Lindahl B, Stenlid J, Olsson S, Finlay R (1999) Translocation of 32P between interacting mycelia of a wood-decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol 144:183–193
Looby CI, Treseder KK (2018) Shifts in soil fungi and extracellular enzyme activity with simulated climate change in a tropical montane cloud forest. Soil Biology and Biochemistry 117:87–96
Marx DH (1972) Ectomycorrhizae as biological deterrents to pathogenic root infections. Annu Rev Phytopathol 10:429–454
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128. https://doi.org/10.1007/s00572-012-0456-9
McGuire KL, Fierer N, Bateman C et al (2012) Fungal community composition in Neotropical rain forests: the influence of tree diversity and precipitation. Microb Ecol 63:804–812. https://doi.org/10.1007/s00248-011-9973-x
Mueller RC, Paula FS, Mirza BS, Rodrigues JLM, Nüsslein K, Bohannan BJM (2014) Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J 8:1548–1550. https://doi.org/10.1038/ismej.2013.253
Neville J, Tessier JL, Morrison I et al (2002) Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-old boreal forest clear-cut. Appl Soil Ecol 19:209–216. https://doi.org/10.1016/S0929-1393(01)00193-7
Nguyen NH, Song Z, Bates ST et al (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Nilsson LO, Giesler R, Bååth E, Wallander H (2005) Growth and biomass of mycorrhizal mycelia in coniferous forests along short natural nutrient gradients. New Phytol 165:613–622. https://doi.org/10.1111/j.1469-8137.2004.01223.x
Oksanen J, Blanchet FG, Friendly M, et al (2017) vegan: Community Ecology Package. R package version 2.4–5. https://CRAN.R-project.org/package=vegan
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011) Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol Lett 14:493–502. https://doi.org/10.1111/j.1461-0248.2011.01611.x
Peay KG, Baraloto C, Fine PV (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. The ISME Journal 7:1852–1861. https://doi.org/10.1038/ismej.2013.66
Põlme S, Bahram M, Yamanaka T et al (2013) Biogeography of ectomycorrhizal fungi associated with alders (Alnus spp.) in relation to biotic and abiotic variables at the global scale. New Phytol 198:1239–1249. https://doi.org/10.1111/nph.12170
Prescott CE, Grayston SJ (2013) Tree species influence on microbial communities in litter and soil: current knowledge and research needs. For Ecol Manag 309:19–27. https://doi.org/10.1016/j.foreco.2013.02.034
Pyke CR, Condit R, Aguilar S, Lao S (2001) Floristic composition across a climatic gradient in a neotropical lowland forest. J Veg Sci 12:553–566. https://doi.org/10.2307/3237007
Rizzo DM, Garbelotto M, Davidson JM et al (2002) Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis 86:205–214. https://doi.org/10.1094/PDIS.2002.86.3.205
Robson AD, Abbott LK (1989) 4 - The Effect of Soil Acidity on Microbial Activity in Soils. In: Robson AD (ed) Soil Acidity and Plant Growth. Academic Press, pp 139–165
Rodríguez-Echeverría S, Teixeira H, Correia M et al (2017) Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol 213:380–390. https://doi.org/10.1111/nph.14122
Schappe T, Albornoz FE, Turner BL et al (2017) The role of soil chemistry and plant neighbourhoods in structuring fungal communities in three Panamanian rainforests. J Ecol 105:569–579. https://doi.org/10.1111/1365-2745.12752
Senechkin IV, van Overbeek LS, van Bruggen AHC (2014) Greater Fusarium wilt suppression after complex than after simple organic amendments as affected by soil pH, total carbon and ammonia-oxidizing bacteria. Appl Soil Ecol 73:148–155. https://doi.org/10.1016/j.apsoil.2013.09.003
Serrano MS, Vita PD, Fernández-Rebollo P, Hernández MES (2012) Calcium fertilizers induce soil suppressiveness to Phytophthora cinnamomi root rot of Quercus ilex. Eur J Plant Pathol 132:271–279. https://doi.org/10.1007/s10658-011-9871-6
Sheldrake M, Rosenstock NP, Revillini D et al (2017) Arbuscular mycorrhizal fungal community composition is altered by long-term litter removal but not litter addition in a lowland tropical forest. New Phytol 214:455–467. https://doi.org/10.1111/nph.14384
Smith SE, Read D (2008) 5 - Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. In: Mycorrhizal Symbiosis (Third Edition). Academic Press, London, pp 145–187
Smith SE, Anderson IC, Smith FA (2015) Mycorrhizal associations and phosphorus acquisition: from cells to ecosystems. Annu Plant Rev 48:409–440
Soudzilovskaia NA, Douma JC, Akhmetzhanova AA et al (2015) Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob Ecol Biogeogr 24:371–382. https://doi.org/10.1111/geb.12272
Spear ER, Coley PD, Kursar TA (2015) Do pathogens limit the distributions of tropical trees across a rainfall gradient? J Ecol 103:165–174. https://doi.org/10.1111/1365-2745.12339
Splivallo R, Ottonello S, Mello A, Karlovsky P (2011) Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol 189:688–699. https://doi.org/10.1111/j.1469-8137.2010.03523.x
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963. https://doi.org/10.1111/j.1365-2435.2008.01402.x
Tedersoo L, Jairus T, Horton BM et al (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490. https://doi.org/10.1111/j.1469-8137.2008.02561.x
Tedersoo L, Sadam A, Zambrano M et al (2010) Low diversity and high host preference of ectomycorrhizal fungi in Western Amazonia, a Neotropical biodiversity hotspot. ISME J 4:465–471. https://doi.org/10.1038/ismej.2009.131
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, … Abarenkov K (2014) Global diversity and geography of soil fungi. Science 346(6213). doi:https://doi.org/10.1126/science.1256688
Toju H, Kishida O, Katayama N, Takagi K (2016) Networks depicting the fine-scale co-occurrences of fungi in soil horizons. PloS one 11:e0165987
Toljander JF, Eberhardt U, Toljander YK et al (2006) Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol 170:873–884. https://doi.org/10.1111/j.1469-8137.2006.01718.x
Turner B, Engelbrecht BJ (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315. https://doi.org/10.1007/s10533-010-9466-x
Turner BL, Romero TE (2009) Short-Term Changes in Extractable Inorganic Nutrients during Storage of Tropical Rain Forest Soils. Soil Science Society of America Journal 73:1972–1979. https://doi.org/10.2136/sssaj2008.0407
van der Heijden MGA, Klironomos JN, Ursic M et al (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Wagg C, Jansa J, Stadler M et al (2011) Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology 92:1303–1313. https://doi.org/10.1890/10-1915.1
Wardle DA, Bardgett RD, Klironomos JN et al (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Werner A, Zadworny M, Idzikowska K (2002) Interaction between Laccaria laccata and Trichoderma virens in co-culture and in the rhizosphere of Pinus sylvestris grown in vitro. Mycorrhiza 12:139–145. https://doi.org/10.1007/s00572-002-0159-8
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18:315–322
Zheng Y, Hu H-W, Guo L-D et al (2017) Dryland forest management alters fungal community composition and decouples assembly of root- and soil-associated fungal communities. Soil Biol Biochem 109:14–22. https://doi.org/10.1016/j.soilbio.2017.01.024
Zuur A, Ieno E, Walker N et al (2009) Mixed effects models and extensions in ecology with R1st edn. Springer-Verlag New York, New York
Acknowledgements
We thank members of the Jones Lab at Oregon State University Department of Botany & Plant Pathology for helpful comments on the manuscript and Dayana Agudo and Aleksandra Bielnicka for laboratory support.
Data Accessibility
Sequencing data are available in GenBank (Bioproject number PRJNA363090). Tree neighborhood, OTU table, root biomass, and soil property data are available in Dryad doi 10.5061/dryad.sc38s.
Funding
Smithsonian Tropical Research Institute (STRI) offered logistical support. Financial support for this work comes from Oregon State University and the National Science Foundation (DEB 1542681), a fellowship from Oregon State University, and an internship from STRI.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 2242 kb)
Rights and permissions
About this article
Cite this article
Schappe, T., Albornoz, F.E., Turner, B.L. et al. Co-occurring Fungal Functional Groups Respond Differently to Tree Neighborhoods and Soil Properties Across Three Tropical Rainforests in Panama. Microb Ecol 79, 675–685 (2020). https://doi.org/10.1007/s00248-019-01446-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01446-z