Abstract
Polybia-MP1 (IDWKKLLDAAKQIL-NH2), a helical peptide extracted from the venom of a Brazilian wasp, has broad-spectrum antimicrobial activities without being hemolytic or cytotoxic. This peptide has also displayed anticancer activity against cancer cell cultures. Despite its high selectivity, MP1 has an unusual low net charge (Q = +2). The aspartic residue (D2) in the N-terminal region plays an important role in its affinity and selectivity; its substitution by asparagine (D2N mutant) led to a less selective peptide. Aiming to explore the importance of this residue for the peptides’ affinity, we compared the zwitterionic and anionic vesicle adsorption activity of Polybia-MP1 versus its D2N mutant and also mastoparan X (MPX). The adsorption, electrostatic, and conformational free energies were assessed by circular dichroism (CD) and fluorescence titrations using large unilamellar vesicles (LUVs) at the same conditions in association with measurement of the zeta potential of LUVs in the presence of the peptides. The adsorption free energies of the peptides, determined from the partition coefficients, indicated higher affinity of MP1 to anionic vesicles compared with the D2N mutant and MPX. The electrostatic and conformational free energies of MP1 in anionic vesicles are less favorable than those found for the D2N mutant and MPX. Therefore, the highest affinity of MP1 to anionic vesicles is likely due to other energetic contributions. The presence of D2 in MP1 makes these energetic components 1.2 and 1.5 kcal/mol more favorable compared with the D2N mutant and MPX, respectively.



Similar content being viewed by others
References
Almeida PF, Ladokhin A, White SH (2012) Hydrogen-bond energetics drive helix formation in membrane interfaces. Biochim Biophys Acta 1818:178–182
Andreu D, Rivas L (1999) Animal antimicrobial peptides: an overview. Biopolymers 47:415–433
Arbuzova A, Schwarz G (1999) Pore-forming action of mastoparan peptides on liposomes: a quantitative analysis. Biochim Biophys Acta 1420:139–152
Blondelle SE, Forood B, Houghten RA, Perez-Paya E (1997) Secondary structure induction in aqueous vs membrane-like environments. Biopolymers 42:489–498
dos Santos Cabrera MP, Costa STB, Souza BM, Palma MS, Ruggiero JR, Ruggiero Neto J (2008) Selectivity in the mechanism of action of antimicrobial mastoparan peptide Polybia-MP1. Eur Biophys J 37:879–891
dos Santos Cabrera MP, Alvares DS, Leite NB, Souza BM, Palma MS, Riske KA, Ruggiero Neto J (2011) New insight into the mechanism of action of wasp mastoparan peptides: lytic activity and clustering observed with giant vesicles. Langmuir 27:10805–10813
dos Santos Cabrera MP, Arcisio-Miranda M, Gorjão R, Leite NB, de Souza BM, Curi R, Procopio J, Ruggiero Neto J, Palma MS (2012) Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry 51:4898–4908
Fairman R, Shoemaker KR, York EJ, Stewart JM, Baldwin RL (1989) Further studies of the helix dipole model: effects of a free α-NH3 + or α-COO− group on helix stability. Proteins: Struct Funct Genetics 5:1–7
Harris F, Dennison SR, Phoenix DA (2009) Anionic peptides from eukaryotic organisms. Curr Protein Pep Sci 10:585–606
Harris F, Dennison SR, Singh J, Phoenix DA (2013) On the selectivity and efficacy of defense peptides with respect to cancer cells. Med Res Rev 33:190–234
Herrmann A, Svangard E, Claeson P, Gullbo J, Bohlin L, Goransson U (2006) Key role of glutamic acid for the cytotoxic activity of the cyclotide cycloviolacin O2. Cell Mol Life Sci 63:235–245
Hunter RJ (1981) Zeta potential in colloid science principles and applications, Chapter 2. Academic, London
Ladokhin AS, White H (1999) Folding of amphipathic helices on membranes: energetics of helix formation by melittin. J Mol Biol 285:1363–1369
Ladokhin AS, White SH (2001) Protein chemistry at membrane interfaces: non-additivity of electrostatic and hydrophobic interactions. J Mol Biol 309:543–552
Ladokhin AS, Jayasinghe S, White SH (2000) How to measure and analyze the tryptophan fluorescence in membrane properly and why bother. Anal Biochem 285:235–245
Ladokhin AS, Fernández-Vidal M, White SH (2010) CD spectroscopy of peptides and proteins bound to large unilamellar vesicles. J Membr Biol 236:247–253
Lau SYM, Taneja AK, Hodges RS (1984) Effect of chain length on the stabilization and formation of two stranded α - helical coiled-coil. J Biol Chem 259:13253–13261
Luo P, Baldwin RL (1997) Mechanism of helix indution by trifluoroethanol: a framework for extrapolationg the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 36:8418–8421
Leite NB, Costa LC, Alvares DS, dos Santos Cabrera MP, Souza BM, Palma MS, Ruggiero Neto J (2011) The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and CD spectroscopy. Amino Acids 40:91–100
Marqusee S, Baldwin RL (1987) Helix stabilization by Glu−… Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci USA 84:8898–8902
Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyajima K (1994) Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry 33:3342–3349
Mckeown AN, Naro JL, Huskin LJ, Almeida PF (2011) A thermodynamic approach to the mechanism of cell-penetrating peptides in model membranes. Biochemistry 50:654–662
Nakajima T, Uzu S, Wakamatsu K, Saito K, Miyazawa T, Yasuhara T, Tsukamoto Y, Fujino M (1986) Amphiphilic peptides in wasp venom. Biopolymers 25:115–121
Palma MS (2006) In: Handbook of biologically active peptides “Insect venom peptides”. Kastin A (ed) Elsevier/Academic, San Diego, pp 409–417
Persson D, Thorén PEG, Nordén B (2001) Penetration-induced aggregation and subsequent dissociation of negatively charged phospholipid vesicles. FEBS Lett 505:307–312
Rohl CA, Baldwin RL (1998) Deciphering rules of helix stability in peptides. Methods Enzymol 295:1–26
Rouser G, Fleischer S, Yamamoto A (1970) Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494–496
Scholtz JM, Qian H, Robbins VH, Baldwin RL (1993) The energetics of ion-pair and hydrogen-bonding interactions in a helical peptide. Biochemistry 32:9668–9676
Schwarz G, Reiter R (2001) Negative cooperativity and aggregation in biphasic binding of mastoparan X peptide to membranes with acidic lipids. Biophys Chem 90:269–277
Schwarz G, Stankowski S, Rizzo V (1986) Thermodynamic analysis of incorporation and aggregation in a membrane. Application to the pore forming peptide alamethicin. Biochim Biophys Acta 861:141–151
Sforça ML, Oyama S Jr, Canduri F, Lorenzi CCB, Pertinhez TA, Konno K, Souza BM, Palma MS, Ruggiero Neto J, de Azevedo WF Jr, Spisni A (2004) How C-terminal carboxyamidation alters the biological activity of peptides from the venom of the Eumenine solitary wasp. Biochemistry 43:5608
Snider C, Jayasinghe S, Hristova K, White SH (2009) MPEx: a tool for exploring membrane proteins. Protein Sci 18:2624–2628. http://blanco.biomol.edu/MPEx
Souza BM, Mendes MA, Santos LD, Marques MR, Cesar LMM, Almeida RNA, Pagnocca FC, Konno K, Palma MS (2005) Structural and functional characterization of two novel peptide toxins isolated from the venom of the social wasp Polybia paulista. Peptides 26:2157–2164
Souza BM, Silva AR, Resende VMF, Arcuri HA, dos Santos Cabrera MP, Ruggiero Neto J, Palma MS (2009) Characterization of two novel polyfunctional mastoparan peptides from the venom of the social wasp Polybia paulista. Peptides 30:1387–1395
Taheri-Araghi S, Ha B-Y (2010) Cationic antimicrobial peptides: a physical basis for their selctive membrane-disrupting activity. Soft Matter 6:1933–1940
Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R (2008) Antitumor effects and cell selectivity and structure-activity relationship of a novel antimicrobial peptide Polybia-MP1. Peptides 29:2320–2327
Wang GS, Li X, Wang Z (2009a) APD2. The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res 37:D933–D937
Wang K, Yan J, Zhang B, Song J, Jia J, Jia P, Wang R (2009b) Novel mode of action of Polybia-MP1 a novel antimicrobial peptide in multidrug resistant leukemic cells. Cancer Lett 278:65–72
White SH, Wimley WC (1999) Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct 28:319–365
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5(10):905–917
Yandek LE, Pokorny A, Almeida PFF (2009) Wasp mastoparans follow the same mechanism as the cell-penetrating peptide transportan 10. Biochemistry 48:7342–7351
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Acknowledgments
J.R.N. acknowledges the financial support from São Paulo Research Foundation (FAPESP, grant # 2011/11640-5). J.R.N. and M.S.P. are researchers of CNPq. N.B.L. has a PhD grant from CAPES, and D.S.A. has a fellowship from São Paulo Research Foundation (FAPESP, grant # 2012/08147-8).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

249_2014_945_MOESM1_ESM.jpg
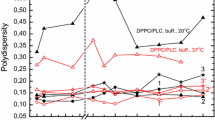
Fig. S1 a Circular dichroism spectra of D2N mutant at 10 μM. Spectra recorded in the absence and in the presence of 0.5 mM of PC/PG (70:30) and PC LUVs in 5 mM Tris-H3BO3, 0.15 M NaF, 0.5 mM Na2EDTA, pH 7.5, at 25 °C. b Hydrodynamic diameters (D H) obtained from the diffusion coefficient (D) using the Stokes–Einstein equation, D H = kT/3πηD, where k is the Boltzmann constant and T is the absolute temperature by dynamic light scattering of the peptide titration. c Molar ellipticity normalized at 222 nm as a function of lipid concentration. D2N-MP1 at 10 µM titrated with zwitterionic (open symbols) and anionic (closed symbols) lipids. Solid lines: nonlinear fit using Eq. 2-b (JPEG 186 kb)

249_2014_945_MOESM2_ESM.jpg
Fig. S2 a Circular dichroism spectra of MPX at 10 μM. Spectra recorded in the absence and in the presence of 0.5 mM of PC/PG (70:30) and PC LUVs in 5 mM Tris-H3BO3, 0.15 M NaF, 0.5 mM Na2EDTA, pH 7.5, at 25 °C. b Hydrodynamic diameters (D H) obtained from the diffusion coefficient (D) using the Stokes–Einstein equation, D H = kT/3πηD, where k is the Boltzmann constant and T is the absolute temperature by dynamic light scattering of the peptide titration. c Molar ellipticity normalized at 222 nm as a function of lipid concentration. MPX at 10 µM titrated with zwitterionic (open symbols) and anionic (closed symbols) lipids. Solid lines: nonlinear fit using Eq. 2-b (JPEG 189 kb)

249_2014_945_MOESM3_ESM.jpg
Fig. S3 a Fluorescence emission spectra of D2N mutant at 5 μM in 5 mM Tris-H3BO3, 0.15 M NaF, 0.5 mM Na2EDTA, pH 7.5, at 25 °C in the presence of PC LUVs, from bottom to top at 325 nm: 0, 0.2, 0.29, 0.38, 0.475, 0.65, 0.9, and 1.3 mM of lipid. b Normalized fluorescence emission intensity as a function of lipid concentration. D2N-MP1 at 5 µM titrated with zwitterionic (open symbols) and anionic (closed symbols) lipids. Solid lines: nonlinear fit using Eq. 4 (JPEG 830 kb)

249_2014_945_MOESM4_ESM.jpg
Fig. S4 a Fluorescence emission spectra of MPX at 5 μM in 5 mM Tris-H3BO3, 0.15 M NaF, 0.5 mM Na2EDTA, pH 7.5, at 25 °C in the presence of PC LUVs, from bottom to top at 325 nm: 0, 0.2, 0.29, 0.38, 0.475, 0.65, 0.9, and 1.3 mM of lipid. b Normalized fluorescence emission intensity as a function of lipid concentration. MPX at 5 µM titrated with zwitterionic (open symbols) and anionic (closed symbols) lipids. Solid lines: nonlinear fit using Eq. 4 (JPEG 813 kb)
Rights and permissions
About this article
Cite this article
Leite, N.B., dos Santos Alvares, D., de Souza, B.M. et al. Effect of the aspartic acid D2 on the affinity of Polybia-MP1 to anionic lipid vesicles. Eur Biophys J 43, 121–130 (2014). https://doi.org/10.1007/s00249-014-0945-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-014-0945-1