Abstract
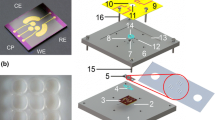
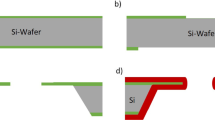
Black lipid membranes (BLMs) have been used for detecting single-channel activities of pore-forming peptides and ion channels. However, the short lifetimes and poor mechanical stability of suspended bilayers limit their applications in high throughput electrophysiological experiments. In this work, we present a synthetic solid-state nanopore functionalized with BLM fused with channel protein. A nanopore with diameter of ~180 nm was electrochemically fabricated in a thin silicon membrane. Folding and painting techniques were demonstrated for production of stable suspended BLMs followed by incorporation of transmembrane protein, ENaC. Membrane formation was confirmed by employing electrochemical impedance spectroscopy (EIS) in the frequency regime of 10−2–105 Hz. Results show that electrochemically fabricated solid state nanopore support resulted in excellent membrane stability, with >1 GΩ of up to 72 and 41 h for painting and folding techniques, respectively. After fusion of ENaC channel protein, the BLM exhibits the stability of ~5 h. We anticipate that such a solid-state nanopore with diameter in the range of 150–200 nm and thickness <1 µm could be a potential platform to enhance the throughput of ion-channel characterization using BLMs.







Similar content being viewed by others
References
Awayda MS, Shao W, Guo F, Zeidel M, Hill WG (2004) ENaC–membrane interactions: regulation of channel activity by membrane order. J Gen Physiol 123(6):709–727
Bally M, Bailey K, Sugihara K, Grieshaber D, Vörös J, Stäler B (2010) Liposome and lipid bilayer arrays towards biosensing applications. Small 6(22):2481–2497
Batishchev OV, Indenbom AV (2008) Alkylated glass partition allows formation of solvent-free lipid bilayer by Montal–Mueller technique. Bioelectrochemistry 74(1):22–25
Benz R, Fröhlich O, Läuger P, Montal M (1975) Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta 394(3):323–334
Berdiev BK, Benos DJ (2006) Epithelial sodium channel in planar lipid bilayers. Methods Mol Biol 337:89–99
Buchholz K, Tinazli A, Kleefen A, Dorfner D, Pedone D, Rant U, Tampé R, Abstreiter G, Tornow M (2008) Silicon-on-insulator based nanopore cavity arrays for lipid membrane investigation. Nanotechnology 19(44):445305
Chang H, Iqbal SM, Stach EA, King AH, Zaluzec NJ, Bashir R (2006) Fabrication and characterization of solid-state nanopores using a field emission scanning electron microscope. Appl Phys Lett 88(10):103109
Cheng Y, Bushby RJ, Evans SD, Knowles PF, Miles RE, Ogier SD (2001) Single ion channel sensitivity in suspended bilayers on micromachined supports. Langmuir 17(4):1240–1242
Drexler J, Steinem C (2003) Pore-suspending lipid bilayers on porous alumina investigated by electrical impedance spectroscopy. J Phys Chem B 107:11245–11254
Gutsmann T, Heimburg T, Keyser U, Mahendran KR, Winterhalter M (2015) Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat Protoc 10(1):188–198
Han X, Studer A, Sehr H, Geissbühler I, Di Berardino M, Winkler FK, Tiefenauer LX (2007) Nanopore arrays for stable and functional free-standing lipid bilayers. Adv Mater 19(24):4466–4470
Hirano-Iwata A, Oshima A, Nasu T, Taira T, Kimura Y, Niwano M (2010a) Stable lipid bilayers based on micro- and nano-fabrication. Supramol Chem 22(7–8):406–412
Hirano-Iwata A, Taira T, Oshima A, Kimura Y, Niwano M (2010b) Improved stability of free-standing lipid bilayers based on nanoporous alumina films. Appl Phys Lett 96(21):213706
Hirano-Iwata A, Aoto K, Oshima A, Taira T, Yamaguchi RT, Kimura Y, Niwano M (2010c) Free-standing lipid bilayers in silicon chips-membrane stabilization based on microfabricated apertures with a nanometer-scale smoothness. Langmuir 26(3):1949–1952
Hirano-Iwata A, Oshima A, Mozumi H, Kimura Y, Niwano M (2012) Stable lipid bilayers based on micro- and nano-fabrication as a platform for recording ion-channel activities. Anal Sci 28(11):1049–1057
Ismailov II, Awayda MS, Berdiev BK, Bubien JK, Lucas JE, Fuller CM, Benos DJ (1996) Triple-barrel organization of ENaC, a cloned epithelial Na+ channel. J Biol Chem 271(2):807–816
Kalsi S, Powl AM, Wallace BA, Morgan H, De Planque MRR (2014) Shaped apertures in photoresist films enhance the lifetime and mechanical stability of suspended lipid bilayers. Biophys J 106(8):1650–1659
Kant K, Kurkuri M, Yu J, Shapter JG, Priest C, Losic D (2014) Impedance spectroscopy study of nanopore arrays for biosensing applications. Sci Adv Mater 6(7):1375–1381
Kataoka-Hamai C, Higuchi M, Iwai H, Miyahara Y (2010) Detergent-mediated formation of polymer-supported phospholipid bilayers. Langmuir 26(18):14600–14605
Kerner Z, Pajkossy T (1998) Impedance of rough capacitive electrodes: the role of surface disorder. J Electroanal Chem 448(1):139–142
Khan M, Williams J (2015) Fabrication of solid state nanopore in thin silicon membrane using low cost multistep chemical etching. Materials 8(11):7389–7400
Khan MS, Dosoky NS, Williams JD (2013) Engineering lipid bilayer membranes for protein studies. Int J Mol Sci 14:21561–21597
Kim CH, Kisiel K, Jung J, Ulanski J, Tondelier D, Geffroy B, Bonnassieux Y, Horowitz G (2012) Persistent photoexcitation effect on the poly(3-hexylthiophene) film: impedance measurement and modeling. Synth Met 162(5–6):460–465
Korman CE, Megens M, Ajo-Franklin CM, Horsley DA (2013) Nanopore-spanning lipid bilayers on silicon nitride membranes that seal and selectively transport ions. Langmuir 29(14):4421–4425
Kresák S, Hianik T, Naumann RLC (2009) Giga-seal solvent-free bilayer lipid membranes: from single nanopores to nanopore arrays. Soft Matter 5(20):4021
Kumar K, Isa L, Egner A, Schmidt R, Textor M, Reimhult E (2011) Formation of nanopore-spanning lipid bilayers through liposome fusion. Langmuir 27(17):10920–10928
Macdonald JR, Barsoukov E (eds) (2005) Impedance spectroscopy: theory, experiment, and applications. Wiley, Hoboken
Mayer M, Kriebel JK, Tosteson MT, Whitesides GM (2003) Microfabricated teflon membranes for low-noise recordings of ion channels in planar lipid bilayers. Biophys J 85(4):2684–2695
Montal M, Mueller P (1972) Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci USA 69(12):3561–3566
Mueller P, Rudin DO, Tien HT, Wescott WC (1962) Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979–980
Naumann CA, Prucker O, Lehmann T, Rühe J, Knoll W, Frank CW (2002) The polymer-supported phospholipid bilayer: tethering as a new approach to substrate-membrane stabilization. Biomacromolecules 3(1):27–35
Naumowicz M, Petelska AD, Figaszewski ZA (2006a) Impedance analysis of a phosphatidylcholine-phosphatidylethanolamine system in bilayer lipid membranes. Electrochim Acta 51(24):5024–5028
Naumowicz M, Kotynska J, Petelska A, Figaszewski Z (2006b) Impedance analysis of phosphatidylcholine membranes modified with valinomycin. Eur Biophys J 35(3):239–246
Nelson N, Anholt R, Lindstrom J, Montal M (1980) Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci USA 77(5):3057–3061
Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5(12):993–996
Pantoja R, Sigg D, Blunck R, Bezanilla F, Heath JR (2001) Bilayer reconstitution of voltage-dependent ion channels using a microfabricated silicon chip. Biophys J 81(4):2389–2394
Peterman MC, Ziebarth JM, Braha O, Bayley H, Fishman HA, Bloom DM (2002) Ion channels and lipid bilayer membranes under high potentials using microfabricated apertures. Biomed Microdevices 4(3):231–236
Phung T, Zhang Y, Dunlop J, Dalziel J (2011) Bilayer lipid membranes supported on Teflon filters: a functional environment for ion channels. Biosens Bioelectron 26(7):3127–3135
Reimhult E, Kumar K (2008) Membrane biosensor platforms using nano- and microporous supports. Trends Biotechnol 26(2):82–89
Römer W, Steinem C (2004) Impedance analysis and single-channel recordings on nano-black lipid membranes based on porous alumina. Biophys J 86(2):955–965
Simon A, Girard-Egrot A, Sauter F, Pudda C, Picollet D’Hahan N, Blum L, Chatelain F, Fuchs A (2007) Formation and stability of a suspended biomimetic lipid bilayer on silicon submicrometer-sized pores. J Colloid Interface Sci 308(2):337–343
Studer A, Han X, Winkler FK, Tiefenauer LX (2009) Formation of individual protein channels in lipid bilayers suspended in nanopores. Colloids Surf B Biointerfaces 73(2):325–331
Tantawi KH, Berdiev B, Cerro R, Williams JD (2013a) Porous silicon membrane for investigation of transmembrane proteins. Superlattices Microstruct 58:72–80
Tantawi KH, Cerro R, Berdiev B, Martin MED, Montes FJ, Patel D, Williams JD (2013b) Investigation of transmembrane protein fused in lipid bilayer membranes supported on porous silicon. J Med Eng Technol 37(1):28–34
Venkatesan BM, Polans J, Comer J, Sridhar S, Wendell D, Aksimentiev A, Bashir R (2011) Lipid bilayer coated Al2O3 nanopore sensors: towards a hybrid biological solid-state nanopore. Biomed Microdevices 13(4):671–682
Vockenroth IK, Atanasova PP, Jenkins ATA, Köper I (2008) Incorporation of alpha-hemolysin in different tethered bilayer lipid membrane architectures. Langmuir 24(2):496–502
Wang XH, Ma S, Su Y, Zhang Y, Bi H (2015) High impedance droplet–solid interface lipid bilayer membranes. Anal Chem 87(4):2094–2099
Weiskopf D, Schmitt EK, Klühr MH, Dertinger SK, Steinem C (2007) Micro-BLMs on highly ordered porous silicon substrates: rupture process and lateral mobility. Langmuir 23(18):9134–9139
Wilk SJ, Goryll M, Laws GM, Goodnick SM, Thornton TJ, Saraniti M, Tang J, Eisenberg RS (2004) Teflon™-coated silicon apertures for supported lipid bilayer membranes. Appl Phys Lett 85(15):3307
Yung-Cheng W, Dau-Chung W, Tsan-Chu L (2014) Using focused electron beams to drill straight nanopores on a membrane. Int J Autom Smart Technol 4(3):157–162
Zagnoni M, Sandison ME, Morgan H (2009) Microfluidic array platform for simultaneous lipid bilayer membrane formation. Biosens Bioelectron 24(5):1235–1240
Zhu Z-W, Wang Y, Zhang X, Sun C-F, Li M-G, Yan J-W, Mao B-W (2012) Electrochemical impedance spectroscopy and atomic force microscopic studies of electrical and mechanical properties of nano-black lipid membranes and size dependence. Langmuir 28(41):14739–14746
Acknowledgments
This work was supported by the Office of the Vice President for Research and Economic Development, University of Alabama in Huntsville (UAH), Huntsville, AL. Dr. Berdiev was supported, in whole or in part, by National Institutes of Health grant R21HL085112, the University of Alabama at Birmingham (UAB) Health Services Foundation General Endowment Fund, and Nazarbayev University Social Policy Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, M.S., Dosoky, N.S., Berdiev, B.K. et al. Electrochemical impedance spectroscopy for black lipid membranes fused with channel protein supported on solid-state nanopore. Eur Biophys J 45, 843–852 (2016). https://doi.org/10.1007/s00249-016-1156-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1156-8