Abstract
The environmental strain Aeromonas salmonicida subsp. pectinolytica 34melT produces abundant melanin through the homogentisate pathway in several culture media, but unexpectedly not when grown in a medium containing glycerol. Using this observation as a starting point, this study investigated the underlying causes of the inhibition of melanin synthesis by glycerol, to shed light on factors that affect melanin production in this microorganism. The effect of different carbon sources on melanin formation was related to the degree of oxidation of their C atoms, as the more reduced substrates delayed melanization more than the more oxidized ones, although only glycerol completely abolished melanin production. Glyphosate, an inhibitor of aromatic amino acid synthesis, did not affect melanization, while bicyclopyrone, an inhibitor of 4-hydroxyphenylpyruvate dioxygenase (Hpd), the enzyme responsible for the synthesis of homogentisate, prevented melanin synthesis. These results showed that melanin production in 34melT depends on the degradation of aromatic amino acids from the growth medium and not on de novo aromatic amino acid synthesis. The presence of glycerol changed the secreted protein profile, but none of the proteins affected could be directly connected with melanin synthesis or transport. Transcription analysis of hpd, encoding the key enzyme for melanin synthesis, showed a clear inhibition caused by glycerol. The results obtained in this work indicate that a significant decrease in the transcription of hpd, together with a more reduced intracellular state, would lead to the abolishment of melanin synthesis observed. The effect of glycerol on melanization can thus be attributed to a combination of metabolic and regulatory effects.
Similar content being viewed by others
Introduction
Homogentisate is a central intermediate in the aromatic amino acid degradation pathway. When this compound accumulates, it oxidizes spontaneously and polymerizes to form a kind of melanin denominated pyomelanin. Aeromonas salmonicida subsp. pectinolytica 34melT is an extremophile that thrives in the waters of a heavily polluted river in Argentina (Pavan et al. 2000). This microorganism produces abundant melanin through the homogentisate pathway (Pavan et al. 2015), a mechanism that has also been described for melanin formation in some bacteria and fungi (Solano 2014). The homogentisate pathway does not occur in the model organism Escherichia coli (McFall and Newman 1996), but it has been extensively studied in Pseudomonas (Arias-Barrau et al. 2004; Rodríguez-Rojas et al. 2009). This pathway is also present in humans and other mammals (Mistry et al. 2013), and mutations in the gene that encodes the enzyme that degrades homogentisate have been observed to result in its accumulation leading to alkaptonuria (Fernández-Cañón et al. 1996; Zatkova 2011).
Melanin has been shown to protect microorganisms from a plethora of environmental stress conditions such as ultraviolet radiation, starvation, high temperature, salinity, oxidative stress, and toxic heavy metals (Plonka and Grabacka 2006; Turick et al. 2010). In addition, melanin can act as electron donor or acceptor having a role in anaerobic metabolism, and also in metal transformations (Turick et al. 2003), highlighting its diverse physiological roles. Recent studies have reported that melanin produced by marine bacterial biofilms can inhibit the settlement of fouling organisms (Zeng et al. 2017), and in Vibrio cholerae, melanin has been recognized as a defensive mechanism against predation (Noorian et al. 2017), demonstrating that this pigment can affect microbial interactions in the environment. In some human microbial pathogens, melanin synthesis has been related to virulence. For example, melanin produced by Burkholderia cenocepacia can scavenge free radicals, resulting in the attenuation of the host cell oxidative burst (Keith et al. 2007), and in the fungus Sporothrix schenckii, pyomelanin makes cells more resistant to nitrosative stress (Almeida-Paes et al. 2012).
Given the multiple roles of melanin on different processes, including environmental fitness, pathogen virulence, and human diseases, it would be important to identify conditions that control homogentisate accumulation. Only a few inhibitors of this pathway are known, including herbicides that inhibit the 4-hydroxyphenylpyruvate dioxygenase currently used to control melanin synthesis in humans (Rocaboy-Faquet et al. 2014; Santucci et al. 2017) and a new compound identified in a high-throughput screening using Legionella pneumophila (Aubi et al. 2015).
Aeromonas salmonicida subsp. pectinolytica is considered to be environmental, in contrast to all the other subspecies, which have been related to fish diseases (Vincent et al. 2017). Melanin production may increase the fitness of 34melT and help it survive under the stressful conditions encountered in extremely polluted river water (Pavan et al. 2015). Colonies of this microorganism are strongly pigmented when grown in rich medium, turning the whole plates a dark brown color due to diffusing melanin. Previous analysis of melanin formation conditions revealed that the pigment was also synthesized in minimal medium when supplemented with tyrosine, especially if copper or iron ions were present (Pavan et al. 2015). However, we unexpectedly noticed that 34melT did not produce melanin when grown in a rich medium containing glycerol routinely used in the lab. This serendipitous observation prompted us to deepen the knowledge of melanin production in this bacterium taking into account that little is known about melanin synthesis control mechanisms.
The aim of this study was to investigate the underlying causes of the inhibition of melanin synthesis by glycerol in A. salmonicida subsp. pectinolytica 34melT, to shed light on factors that affect melanin production in this microorganism.
Materials and methods
Bacterial strain and growth conditions
Aeromonas salmonicida subsp. pectinolytica 34melT (DSM 12609T) was grown at 28 °C either in Terrific Broth (Invitrogen, Carlsbad, USA) containing 1% (w/v) glycerol (0.11 M) or in lysogeny broth (LB) medium. Melanin synthesis was analyzed in LB agar plates supplemented with glycerol 0.5, 0.75 or 1% (w/v), 0.05 M d-glucose, 0.05 M d-mannitol, 0.05 M sodium d-gluconate, 0.11 M sodium pyruvate, or 0.11 M sodium l-lactate, as indicated in each case. Plates were observed daily for melanin production. To analyze the role of the 4-hydroxyphenylpyruvate dioxygenase on melanin production, the specific inhibitor bicyclopyrone [4-hydroxy-3-(2-(2-methoxy-ethoxymethyl)-6-trifluoromethyl-pyridine-3-carbonyl)-bicyclo(3,2,1) oct-3-en-2-one] was added to the growth medium at 10 μM, 100 μM, 1 mM, and 10 mM final concentration. The aromatic amino acid synthesis inhibitor glyphosate [N-(phosphonomethyl)glycine] was used at concentrations ranging from 10 μM to 25 mM.
Analysis of secreted proteins by mass spectrometry
Cultures grown in LB for 4 days at 28 °C and 300 rpm, with or without the addition of 1% glycerol, were centrifuged and supernatants filtered through a 0.2-μm pore size filter in order to eliminate cells. Supernatants were concentrated 80 times using Amicon Ultra-0.5 ml Centrifugal Filters 10 kDa (Millipore, Burlington, USA), quantified using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, USA), and analyzed by 15% SDS-PAGE. The gel was lightly stained with Brilliant Blue R (Sigma-Aldrich, St. Louis, USA) to individualize dominant protein bands that were neatly excised from the gel. Protein digestion and mass spectrometry (MS) analysis were performed at the Proteomics Core Facility CEQUIBIEM, at the University of Buenos Aires as follows: excised protein bands were sequentially washed and destained with 50 mM ammonium bicarbonate, 25 mM ammonium bicarbonate 50% acetonitrile, and 100% acetonitrile; reduced and alkylated with 10 mM dithiothreitol and 20 mM iodoacetamide and in-gel digested with 100 ng Trypsin (Cat. No. V5111, Promega, Madison, USA) in 25 mM ammonium bicarbonate overnight at 37 °C. Peptides were recovered by elution with 50% acetonitrile − 0.5% trifluoroacetic acid, including brief sonication, and further concentrated by speed-vacuum drying. Samples were resuspended in 15 μl of water containing 0.1% formic acid. Peptides were analyzed in a UV-MALDI-TOF/TOF (Ultraflex II, Bruker Daltonics Inc., Billerica, USA). The software used for spectra visualization and MS/MSMS protein identification were Flex Analysis (v. 3.3) and BioTools (Bruker Daltonics Inc., Billerica, USA), linked to MASCOT (Matrix Science, Boston, USA, 2016, http://www.matrixscience.com/), to search against the NCBInr protein sequence databases.
GlpR binding motif search
To determine the presence of GlpR binding sites, consensus sequences of motifs previously identified in Vibrionaceae, Pseudomonadaceae, and Enterobacteriaceae (Danilova et al. 2003) were computationally searched within the neighborhood of the genes of interest (400 bp upstream and 300 bp downstream from coding sequences). Local alignments between published binding motifs and 34melT nucleotide sequences were performed using the Bioinformatics Toolbox available in Matlab (Mathworks, Natick, USA), with an identity threshold of 65%.
Transcriptional analysis of hpd
Total RNA of 34melT was extracted from 3 ml of 24 h aerobic cultures grown at 28 °C in LB and LB plus 1% (w/v) glycerol using the Total RNA Extraction Kit (RBC Biosciences, New Taipei City, Taiwan). Seven independent cultures were used for each condition. After treatment with DNaseI, total RNA was converted into cDNA using random hexamers (Promega, Madison, USA) and Revert Aid Reverse Transcriptase (Thermo Fisher Scientific, Waltham, USA) following the manufacturer’s instructions. The cDNA was quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, USA), and used to detect the transcription of hpd in both culture conditions. Expression of the 16S rRNA gene was used as control. The primers hpd forward 5′-TCTACCAGACCATACGCACC and reverse 5′-TATGACGGTGTCGGTGAAGA and 16S rRNA gene forward 5′-CGGGTGAGTAATGCCTGGG and reverse 5′-GTGAGCCATTACCCCACCAA were used, allowing the amplification of 197 bp and 162 bp fragments, respectively. A PCR assay was carried out in a SimpliAmp Thermal Cycler (Thermo Fisher Scientific, Waltham, USA) using One Taq DNA Polymerase (New England BioLabs Inc., Ipswich, USA), 0.3 μM of the corresponding pair of primers, and serial dilutions of the cDNA as template. The cycling conditions were as follows: 95 °C for 8 min, 35 cycles at 95 °C for 20 s, 60 °C for 20 s, and 68 °C for 25 s, followed by 68 °C for 8 min. PCR products were visualized by 1.5% agarose gel electrophoresis. A 100-bp DNA ladder was used as molecular size standard (Genbiotech, Buenos Aires, Argentina).
Quantitative real-time reverse-transcriptase PCR (qRT-PCR) experiments were conducted using the MyiQ2 Two-Color Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, USA). Each reaction was performed using EvaGreen qPCR Mix Plus no ROX (Solis Biodyne, Tartu, Estonia), 0.5 μM of each primer, and 2 μl of serial dilutions of cDNA. The cycling conditions were as follows: 95 °C for 15 min, 38 cycles at 95 °C for 25 s, 60 °C for 15 s, and 72 °C for 20 s, with fluorescence acquisition at 80 °C in single mode. Relative changes in the expression of genes in cultures with and without glycerol were obtained through the relative standard curve method (Larionov et al. 2005). The difference of hpd expression between both culture conditions was evaluated by the Student’s t test with confidence levels at > 95% in which P < 0.05 was considered as statistically significant.
Results
Glycerol affects melanin production
Colonies of 34melT grown in lysogeny broth (LB) agar plates are strongly pigmented, displaying a dark brown color that diffuses to the surrounding medium in approximately 24 h. When this microorganism was plated in Terrific Broth (TB) containing 1% glycerol, colony formation was vigorous, but melanization only started after several days. This observation was surprising because some amino acids such as tyrosine have been shown to enhance melanin production in this bacterium (Pavan et al. 2015), and TB contains higher amounts of amino acids than LB. Since TB contains glycerol, melanin synthesis was analyzed in LB supplemented with different glycerol concentrations in order to investigate if this compound was responsible for the change observed in melanin synthesis. While in LB, colonies started to show pigmentation after 24 h, plates containing 0.5% glycerol developed pigmentation only after 2 days, and no melanin formation was observed in LB plates containing 0.75% or 1% glycerol (Fig. 1) even after prolonged incubation (30 days). These results demonstrated that the delay in melanin production observed in TB could be attributed to the effect of glycerol. Furthermore, the presence of the highest concentrations of glycerol inhibited melanin production in LB completely.
Effect of different carbon sources on melanization
Since melanin in 34melT is formed by the oxidation and polymerization of homogentisate, produced from tyrosine, its synthesis could potentially be affected by growth conditions that alter either the redox state of the cells or central carbon metabolism, including aromatic amino acid metabolism. To investigate these possibilities, the addition of several carbon sources was tested.
Melanin formation was analyzed in LB supplemented with different substrates containing C atoms with increasing degrees of oxidation: glycerol, mannitol, glucose, and gluconate. Glycerol was used at 1% (0.11 M) final concentration, while 0.05 M of the six carbon substrates was added to ensure the same molar amount of carbon in each case. Melanin production was observed in LB plates after overnight incubation, was delayed for a few hours in the presence of gluconate, and started after 2 days when glucose was added to the medium. Colonies in plates containing mannitol delayed melanization slightly more than glucose, and as expected, no melanin was observed in plates with glycerol. After several days, colonies in plates supplemented with these additives finally reached the same level of pigmentation observed in LB medium, except for plates containing glycerol that did not melanize, although growth was similar in all conditions. These results showed that the addition of all carbon sources delayed melanization, suggesting that the C/N relationship of the medium had a minor effect on melanin synthesis. The effect observed for each substrate was related to the degree of oxidation of its C atoms, as the more reduced substrates delayed melanin formation more than the more oxidized ones. However, the only substrate that completely prevented melanization was glycerol.
Since glycerol can be a substrate for gluconeogenesis, the effect on melanin production of pyruvate and lactate, other known gluconeogenic carbon sources, was investigated. There were no differences in pigment development in LB medium with the addition of 0.11 M pyruvate or lactate when compared to LB, although growth was delayed nearly 24 h in the presence of lactate. As before, melanin was not formed at any time in plates containing 0.11 M glycerol. These results indicated that only glycerol and not other gluconeogenic carbon sources inhibited melanization.
Glycerol metabolism genes
Analysis of the genome of 34melT (Pavan et al. 2015) revealed a cluster of six genes related to the transport and utilization of glycerol in this microorganism (Fig. 2a). Two genes, glpF (K931_04197) that encodes an aquaglyceroporin permeable to glycerol as well as water in E. coli (Chen 2013) and glpK (K931_04192) that encodes a glycerol kinase, are transcribed in the opposite direction from the rest of the genes. The gene encoding the glycerol-3-phosphate transporter GlpT (K931_04202) is followed by glpR (K931_04207) encoding the regulon repressor, glpD (K931_04217) corresponding to the glycerol-3-phosphate dehydrogenase, and finally by glpQ (K931_04222) that encodes a periplasmic glycerophosphodiester phosphodiesterase. A thorough search for all genes related to glycerol metabolism in 34melT showed the presence of two additional gene clusters containing homologs to genes involved in anaerobic glycerol metabolism in E. coli (Gonzalez et al. 2008).
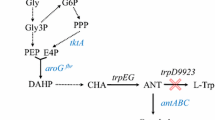
Organization of the glp cluster and melanin synthesis pathway. aglp cluster of 34melT showing the location of aroF and tyrA. Genes are colored according to their function: light blue, transport; blue, glycerol utilization; violet, transcriptional regulator; green; aromatic amino acid metabolism. bDe novo synthesis and degradation of aromatic amino acids and melanin formation. PEP, phosphoenolpyruvate; AroF, 3-deoxy-7-phosphoheptulonate synthase; AroA, 5-enolpyruvylshikimate-3-phosphate synthase; TyrA, bifunctional chorismate synthase/prephenate dehydrogenase; PhhA, phenylalanine 4-monooxygenase; PhhB, pterin-4-α-carbinolamine dehydratase; PhhC, aromatic amino acid aminotransferase; Hpd, 4-hydroxyphenylpyruvate dioxygenase; HmgA, homogentisate 1,2-dioxygenase; IS, insertion sequence
Interestingly, genes encoding the 3-deoxy-7-phosphoheptulonate synthase AroF (K931_04227) and the bifunctional chorismate mutase/prephenate dehydrogenase TyrA (K931_04232), both involved in aromatic amino acid metabolism (Bongaerts et al. 2001) (Fig. 2b), are located adjacent to the glp cluster (Fig. 2a), an organization that is conserved in Aeromonas. Analysis of the genomic location of aroF and tyrA in other bacteria in which the homogentisate pathway has been described, such as Pseudomonas putida, revealed that these genes are located in different regions of the genome, and not in the vicinity of glp genes (data not shown).
Inhibition of melanin synthesis by glycerol is not dependent on de novo aromatic amino acid synthesis
The next step was to investigate which stages of melanin production could be affected by glycerol. Melanin synthesis in 34melT has been proposed to occur through the homogentisate pathway based on genome analysis (Pavan et al. 2015). To experimentally corroborate that melanin is a product of aromatic amino acid degradation, the effect of bicyclopyrone on melanin synthesis was investigated. Bicyclopyrone is an inhibitor of the 4-hydroxyphenylpyruvate dioxygenase Hpd, the enzyme that catalyzes the conversion of 4-hydroxyphenylpyruvate to homogentisate is the last step in the melanin biosynthesis pathway (Fig. 2b).
Cultures were grown in LB supplemented with increasing concentrations of bicyclopyrone. This compound had no effect on growth except for a slight inhibition at 10 mM. At the lowest concentration tested (10 μM), pigment production was delayed and did not reach the same level as the control even after 3 days, while melanin synthesis was completely abolished at 100 μM bicyclopyrone (Fig. 3). These results clearly show that Hpd activity is essential for melanization in 34melT. When the experiment was repeated with 1% glycerol, no melanization was observed independently of the inhibitor concentration.
As indicated above, the glp cluster in 34melT is adjacent to aroF and tyrA, an organization unique to Aeromonas. Since both genes are involved in de novo aromatic amino acid synthesis (Fig. 2b), a possible relationship of melanin formation with this pathway was investigated. Melanin production was monitored in the presence of glyphosate, a compound that inhibits the condensation of shikimate 3-phosphate and phosphoenolpyruvate to produce a precursor of chorismate, a central metabolite leading to aromatic amino acid synthesis (Fig. 2b).
Cells were grown in LB supplemented with concentrations of glyphosate ranging from 10 μM to 25 mM. Growth was observed in glyphosate concentrations up to 1 mM, and all cultures developed pigmentation, indicating that the presence of glyphosate did not affect melanin synthesis (data not shown). These results clearly show that melanin production does not depend on de novo aromatic amino acid synthesis in 34melT. When the experiment was performed in the presence of 1% glycerol, no melanin formation was observed regardless of the presence of glyphosate, indicating that glycerol affects melanin formation from aromatic amino acids provided by the growth medium.
Glycerol affects the secreted protein pattern
Changes in extracellular proteins, which can include those involved in transport, were analyzed to investigate possible associations with differences in pigment production in LB cultures with and without glycerol. After 4 days, the LB cultures were intensely melanized, while no melanin was observed in those supplemented with glycerol. The final pH of the LB cultures was 8, and those with glycerol had a pH of around 5. Major protein bands observed in polyacrylamide gels were identified by mass spectrometry. Five dominant protein bands were detected in LB supernatants and six in those of LB plus glycerol, three of which were present in both conditions (Fig. 4, Table S1). Two bands that were detected only in LB, and not in the presence of glycerol, were identified as the peptide ABC transporter substrate-binding protein DppA and the outer membrane protein OmpK. On the other hand, three bands were only observed in glycerol cultures. These corresponded to the aerolysin AerA, a glycerophospholipid-cholesterol acyltransferase commonly known as GCAT, and the outer membrane protein OmpAI.
Extracellular proteins observed in cultures grown in LB and LB supplemented with glycerol. Cultures were grown at 28 °C with agitation during 4 days. a SDS-PAGE. b Description of the major bands identified by mass spectrometry, with the locus tags of the corresponding genes. The X indicates presence of each protein
To study the possible relationship between the peptide ABC transporter DppA, glycerol and melanin production, the presence of this protein was analyzed in the supernatants of cultures grown in LB and LB plus glycerol, with and without 100 μM bicyclopyrone. The band corresponding to DppA, corroborated by mass spectrometry, was observed in supernatants of cultures grown in LB, that were intensely melanized, but also in the cultures with bicyclopyrone, that did not develop pigmentation (data not shown). As before, no DppA was observed in LB glycerol cultures independently of the presence of bicyclopyrone. These results indicate that the lack of melanin observed in cultures with glycerol is not related to the absence of DppA.
Glycerol inhibits transcription of hpd
As indicated in the previous section, the presence of glycerol affected the secreted protein profile, suggesting that it changes protein expression in 34melT, so the possibility that glycerol could affect the expression of the genes involved in aromatic amino acid degradation was investigated.
Analysis of the genome of 34melT showed a cluster that contains phhA (K931_10868), phhB (K931_10863), and tyrR (K931_10858), that along with two copies of phhC (K931_06321, K931_11063) are involved in the transformation of phenylalanine into tyrosine and then to 4-hydroxyphenylpyruvate. This compound is further transformed to homogentisate by the enzyme coded by hpd (K931_20202) located upstream from hmgA, that encodes the homogentisate dioxygenase. This last gene is interrupted by an insertion sequence that results in homogentisate accumulation (Pavan et al. 2015) (Fig. 2b).
Glycerol metabolism genes are regulated by the specific regulator GlpR, a repressor that binds its targets in the absence of glycerol (Escapa et al. 2013). As a first approach to analyze the possibility that glycerol could affect the transcription of genes related to melanin synthesis, putative GlpR binding sites were searched in the vicinity of the genes involved in aromatic amino acid degradation. GlpR binding consensus motifs were identified upstream from glpK, glpF, glpT, glpD, and glpQ, validating this approach. GlpR binding motifs were found upstream from the coding sequences of phhB, phhA, both copies of phhC, hpd, and tyrR. However, the repression of aromatic amino acid degradation genes by the binding of GlpR to these potential targets in the presence of glycerol would be in conflict with the known mode of action of this regulator.
Since our results with bicyclopyrone showed that inhibition of Hpd abolished melanization in 34melT, the expression of the corresponding gene was analyzed in the presence and absence of glycerol as a final approach to evaluate possible transcriptional effects of glycerol that could affect melanin production.
The transcription of hpd was first analyzed by reverse transcriptase PCR (RT-PCR) using several dilutions of the cDNA obtained from total RNA of 34melT grown for 24 h in LB with or without glycerol. A clear difference in hpd mRNA levels between both conditions was observed, as a strong band was detected in melanized LB cultures, while only a very faint band was observed in unpigmented glycerol cultures (Fig. 5a). Further analysis using quantitative real-time reverse-transcriptase PCR (qRT-PCR) showed a significantly (P = 0.0002) lower expression of hpd in the presence of glycerol, that was on average 5.2 times lower than in LB cultures (Fig. 5b). This transcriptional analysis demonstrated that hpd expression is strongly affected by the presence of glycerol, and correlates with the differences observed in melanin production.
Effect of glycerol on hpd expression. a Semiquantitative RT-PCR of hpd performed using serial dilutions of cDNA obtained from 24 h LB cultures grown with or without glycerol. The 16S rRNA gene was used as control. M, 100 bp DNA ladder. b Gene expression was quantified by qRT-PCR analysis. Bars represent averages ± standard errors of seven independent experiments. * Significant differences, P = 0.0002
Discussion
Homogentisate is a central intermediate in the catabolism of phenylalanine and tyrosine (Morales et al. 2004). Different alterations in this pathway can lead to homogentisate accumulation that is oxidized outside the cells and polymerized as melanin (Ryan et al. 2014). In some cases, accumulation of this compound is caused by an increase in the expression of Hpd that converts 4-hydroxyphenylpyruvate to homogentisate (Kotob et al. 1995), in others, it is caused by the absence of HmgA (Fernández-Cañón et al. 1996), the enzyme that leads to homogentisate degradation, or to a decreased activity of this last enzyme (Sanchez-Amat et al. 1998). Based on genomic analysis, the synthesis of melanin in A. salmonicida subsp. pectinolytica 34melT has been proposed to occur through the homogentisate pathway, due to an insertion that inactivates hmgA (Pavan et al. 2015). The lack of melanin synthesis observed when this microorganism was grown in the presence of bicyclopyrone, an inhibitor of Hpd, provides conclusive experimental evidence that melanin is produced through aromatic amino acid degradation, and that Hpd is essential for melanin formation. This result is in agreement with the lack of melanin accumulation observed in hpd mutants in Aeromonas media (Wang et al. 2015).
Colonies of 34melT rapidly develop a strong dark brown pigmentation in LB medium, but not when grown in the presence of 1% glycerol. This observation prompted us to investigate different factors that could explain the inhibition of melanin production in glycerol containing medium.
Glycerol is a gluconeogenic substrate, so it was possible that the inhibition of melanin formation caused by glycerol could be due to alterations in central carbon fluxes. However, the addition of the gluconeogenic substrates pyruvate or lactate did not affect melanin synthesis in 34melT, indicating that the inhibition of glycerol cannot be attributed to this trait.
Since homogentisate must be oxidized prior to its polymerization (Ryan et al. 2014), it was hypothesized that glycerol metabolism, that normally leads to a reduced intracellular state (de Almeida et al. 2010), could affect homogentisate oxidation, thus preventing melanin formation. When cells were grown using substrates containing C atoms with increasing oxidation degrees (glycerol, mannitol, glucose, and gluconate), all carbon sources were observed to delay melanization, but only glycerol abolished it completely. Different bacteria are known to have regulatory systems that prioritize the utilization of preferred substrates (Rojo 2010). It is possible that the use of more favorable carbon sources could delay the degradation of aromatic amino acids in 34melT, thus affecting melanin synthesis. The information available on the effect of carbon sources on melanin formation is very scarce. A study conducted in Shewanella showed that glucose caused a reduction in the amount of melanin formed, and attributed this to catabolite repression (Fuqua and Weiner 1993). However, catabolite repression alone would not explain why glycerol exerts a greater inhibition on melanin synthesis in 34melT than all other carbon sources. The effect observed with mannitol, glucose, and gluconate was related to the degree of oxidation of their C atoms, as the delay on melanization produced by the more reduced substrates was more pronounced. Early studies performed with purified homogentisate showed that the reducing agents ascorbic acid, NADH, and reduced glutathione suppressed the oxidation of homogentisate (Martin and Batkoff 1987). Bacteria maintain homogentisate in the quinol form prior to its secretion to prevent intracellular polymerization using NAD(P)H-dependent quinone oxidoreductases (Ryan et al. 2014), so it is possible that a more reduced intracellular environment could delay homogentisate oxidation. However, since all other carbon sources had a partial effect on melanin formation, it is unlikely that the complete inhibition of melanin synthesis observed in 34melT grown in glycerol medium can be attributed solely to a decrease in the oxidation of homogentisate.
Analysis of genes involved in glycerol utilization in 34melT revealed that aroF and tyrA are located adjacent to the glpKFTRDQ cluster. Since AroF and TyrA are involved in aromatic amino acid synthesis (Bongaerts et al. 2001), the possibility that glycerol could affect melanization through the inhibition of this pathway was investigated. AroF catalyzes the first step in the de novo aromatic amino acid synthesis, while TyrA transforms chorismate to prephenate (Bongaerts et al. 2001), and this compound to 4-hydroxyphenylpyruvate, the precursor of both tyrosine and homogentisate (Morales et al. 2004). However, our results showed that blocking the synthesis of chorismate with glyphosate did not affect melanization, demonstrating that melanin production does not depend on de novo aromatic amino acid synthesis. This is further supported by the fact that 34melT does not produce melanin in mineral medium unless tyrosine is added (Pavan et al. 2015). Although we cannot rule out that glycerol could affect aroF or tyrA expression and/or other steps of aromatic amino acid synthesis, the results obtained with glyphosate indicate that the effect of glycerol on melanin formation would not depend on AroF or TyrA activity.
The genetic determinants that affect melanin synthesis have been studied in A. media, Pseudomonas aeruginosa, and Ralstonia solanacearum, revealing that mutations in genes involved in aromatic amino acid degradation, but also transcriptional regulators and transport proteins either abolish or reduce pigment production (Hunter and Newman 2010; Wang et al. 2015; Ahmad et al. 2016). In A. media, the only mutation that inhibited melanization completely was the deletion of hpd, while mutations in other genes of the homogentisate pathway only reduced pigment production (Wang et al. 2015). Transposon insertions in hpd and also in several genetic regulators were observed to abolish melanin synthesis in R. solanacearum (Ahmad et al. 2016). In P. aeruginosa, melanization was completely inhibited in strains containing transposon mutations that affected all the genes related to the homogentisate pathway, a gene encoding an outer membrane protein, and two other that encode proteins of unknown function, while additional mutations affecting nucleotide biosynthesis, transcriptional regulators, or membrane proteins displayed reduced pigment formation (Hunter and Newman 2010). One of these mutations affects an ABC transporter proposed to participate in homogentisate transport (Hunter and Newman 2010). The results obtained with these bacteria suggested that other factors apart from the genes involved in aromatic amino acid degradation could be related to melanin production in 34melT, and could thus contribute to the effect exerted by glycerol. The presence of glycerol caused differences in the secreted protein pattern of 34melT, so the possible relationship of the main proteins observed in each condition with melanin production was analyzed. One of the proteins detected only in LB, and not in the presence of glycerol, was the peptide ABC transporter substrate-binding protein DppA. However, this protein was also observed in LB cultures containing bicyclopyrone that did not produce melanin, so the absence of DppA cannot be related to the lack of melanin formation observed in the presence of glycerol in 34melT. In A. media, DppA was initially related to melanin, but further studies demonstrated that deletion of the corresponding gene did not affect melanization (Chai et al. 2012). Another protein found only in LB corresponds to OmpK, a major outer membrane protein in A. salmonicida A449 (Ebanks et al. 2005). This protein is similar to Tsx, a nucleoside-specific channel-forming protein that is induced in basic conditions in E. coli (Wu et al. 2009), so its absence could be related to a lower pH observed in stationary phase cultures grown with glycerol. On the other hand, three proteins were only detected in cultures supplemented with glycerol. These corresponded to the aerolysin AerA, the glycerophospholipid-cholesterol acyltransferase (GCAT) (Upton and Buckley 1995), and the outer membrane protein OmpAI, that was related to virulence in A. hydrophila (Yu et al. 2005). These proteins were also abundant in the exoproteome of a virulent strain of A. salmonicida subsp. salmonicida (Vanden Bergh et al. 2013). Although the environmental 34melT is considered non-pathogenic and lacks the Type III secretion system required for virulence in A. salmonicida (Stuber et al. 2003; Tanaka et al. 2017), it was surprising that AerA, GCAT, and OmpAI expressed in 34melT in non-melanizing conditions were not observed in conditions that favor pigmentation, since melanin has been shown to contribute to virulence in many microorganisms (Nosanchuk and Casadevall 2003). However, this relationship would not be universal, as a dissociation between melanin production and virulence was reported in Aspergillus in a murine infection model (Keller et al. 2011).
None of the secreted proteins affected by the presence of glycerol could be directly connected with melanin synthesis or transport, but the changes observed suggest that glycerol affects protein synthesis in 34melT, so it is possible that its presence could change the expression of genes involved in melanin production. Several studies performed in other bacteria have reported changes in the transcription of multiple genes, including some involved in aromatic amino acid metabolism, in glycerol containing medium. For example, the presence of glycerol was observed to affect the transcription of aroC in E. coli (Arunasri et al. 2014) and of aroF in P. putida (Nikel et al. 2014). However, the mechanism through which glycerol affects the transcription of these genes has not been elucidated.
Several regulators have been observed to control aromatic amino acid degradation in bacteria. In Pseudomonas, PhhR activates phhA, hpd, and hmgA in the presence of phenylalanine or tyrosine (Herrera et al. 2009; Palmer et al. 2010), and HmgR represses hmgA in P. putida (Arias-Barrau et al. 2004). An in silico study has proposed that HmgR could also control hpd in Shewanella (Stepanova and Rodionov 2011), while in Sinorhizobium meliloti hpd is activated by the specific regulator HpdR (Loprasert et al. 2007). However, the genetic organization of these genes has been observed to differ greatly in different bacteria (Arias-Barrau et al. 2004), so it is difficult to extrapolate the results obtained in these bacteria to other microorganisms. Analysis of the genome of 34melT to search for these regulators revealed that the only one present was TyrR, an orthologous of PhhR. Furthermore, putative TyrR binding sites were found upstream of the coding sequence of phhA and hpd, suggesting that in 34melT, these genes could be under the control of TyrR, as described in Pseudomonas. However, to the best of our knowledge, none of these regulators has been related to glycerol, so there is no information that could shed light on possible relationships between the regulation of the homogentisate pathway genes and the inhibition of melanin synthesis caused by glycerol.
The only known specific regulator associated to glycerol metabolism is GlpR, the repressor of glp genes (Escapa et al. 2013). The search for GlpR binding motifs in 34melT revealed the presence of putative GlpR boxes in the vicinity of phhA, phhB, both copies of phhC, and hpd. However, the proposed mode of action of GlpR, based on studies performed in E. coli and Pseudomonas, is to repress transcription by binding to DNA in the absence of glycerol (Larson et al. 1992; Escapa et al. 2013). In view of this information, it is difficult to hypothesize that in 34melT, GlpR could repress aromatic amino acid degradation genes by binding to these putative GlpR boxes in the presence of glycerol, but a possible effect of glycerol on the expression of these genes by a yet undiscovered mechanism cannot be ruled out.
Analysis of the genetic determinants of melanin synthesis from homogentisate in different microorganisms has revealed that some genes shown to participate in melanin synthesis in some bacteria were not relevant for melanin formation in other microorganisms. However, inactivation of hpd always resulted in abolishment of melanin synthesis in all melanin-producing bacteria analyzed (Hunter and Newman 2010; Wang et al. 2015; Ahmad et al. 2016). Since hpd is essential for melanin synthesis in other microorganisms and Hpd is necessary for melanization in 34melT, the possibility that the effect of glycerol could be exerted through the modulation of hpd expression was investigated. Our transcription analysis demonstrated that the presence of glycerol caused a clear (more than fivefold) inhibition of hpd when compared to cultures grown without glycerol. A study performed in V. cholerae has demonstrated that the capability to produce melanin is based on differences in the activity levels of the enzymes involved, such as a sevenfold difference in the activity of HmgA observed in melanizing vs non melanizing strains (Sanchez-Amat et al. 1998). Taking into account the results obtained in V. cholerae, it is feasible to propose that the decrease in the expression of hpd in 34melT could have a dramatic effect on melanin synthesis even when transcription of this gene was not suppressed in the presence of glycerol. Although we cannot rule out the contribution of other factors, this result indicates that the effect of glycerol on melanization can be related to a decreased hpd expression.
The multi-pronged approach used in this study led to the conclusion that the inhibition of melanin synthesis in the presence of glycerol can be attributed to a combination of metabolic and regulatory effects: i) the generation of a more reduced environment, unfavorable for homogentisate oxidation, and ii) a significant decrease in the transcription of hpd, encoding the key enzyme for melanin formation. Knowledge of the conditions that affect the production of homogentisate in bacteria is expected to shed light on metabolic and/or genetic regulation leading to the accumulation of this compound in other organisms, and could constitute a starting point to design new strategies to control homogentisate accumulation in pathogenic bacteria and humans.
References
Ahmad S, Lee SY, Kong HG, Jo EJ, Choi HK, Khan R, Lee SW (2016) Genetic determinants for pyomelanin production and its protective effect against oxidative stress in Ralstonia solanacearum. PLoS One 11:e0160845. https://doi.org/10.1371/journal.pone.0160845
Almeida-Paes R, Frases S, de Sousa Araújo G, de Oliveira MM, Gerfen GJ, Nosanchuk JD, Zancopé-Oliveira RM (2012) Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl Environ Microbiol 78:8623–8630. https://doi.org/10.1128/AEM.02414-12
Arias-Barrau E, Olivera ER, Luengo JM, Fernández C, Galán B, García JL, Díaz E, Miñambres B (2004) The homogentisate pathway: a central catabolic pathway involved in the degradation of L-phenylalanine, L-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J Bacteriol 186:5062–5077. https://doi.org/10.1128/JB.186.15.5062-5077.2004
Arunasri K, Adil M, Khan PA, Shivaji S (2014) Global gene expression analysis of long-term stationary phase effects in E. coli K12 MG1655. PLoS One 9:e96701. https://doi.org/10.1371/journal.pone.0096701
Aubi O, Flydal MI, Zheng H, Skjærven L, Rekand I, Leiros HK, Haug BE, Cianciotto NP, Martinez A, Underhaug J (2015) Discovery of a specific inhibitor of pyomelanin synthesis in Legionella pneumophila. J Med Chem 58:8402–8412. https://doi.org/10.1021/acs.jmedchem.5b01589
Bongaerts J, Krämer M, Müller U, Raeven L, Wubbolts M (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3:289–300. https://doi.org/10.1006/mben.2001.0196
Chai B, Wang H, Chen X (2012) Draft genome sequence of high-melanin-yielding Aeromonas media strain WS. J Bacteriol 194:6693–6694. https://doi.org/10.1128/JB.01807-12
Chen LY (2013) Glycerol modulates water permeation through Escherichia coli aquaglyceroporin GlpF. Biochim Biophys Acta 1828:1786–1793. https://doi.org/10.1016/j.bbamem.2013.03.008
Danilova LV, Gelfand MS, Lyubetsky VA, Laikova ON (2003) Computer-assisted analysis of regulation of the glycerol-3-phosphate metabolism in genomes of proteobacteria. Mol Biol 37:716–722. https://doi.org/10.1023/A:1026037027266
de Almeida A, Giordano AM, Nikel PI, Pettinari MJ (2010) Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichia coli. Appl Environ Microbiol 76:2036–2040. https://doi.org/10.1128/AEM.02706-09
Ebanks RO, Goguen M, McKinnon S, Pinto DM, Ross NW (2005) Identification of the major outer membrane proteins of Aeromonas salmonicida. Dis Aquat Org 68:29–38. https://doi.org/10.3354/dao068029
Escapa IF, del Cerro C, García JL, Prieto MA (2013) The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol 15:93–110. https://doi.org/10.1111/j.1462-2920.2012.02790.x
Fernández-Cañón JM, Granadino B, Beltrán-Valero de Bernabé D, Renedo M, Fernández-Ruiz E, Peñalva MA, Rodríguez de Córdoba S (1996) The molecular basis of alkaptonuria. Nat Genet 14:19–24. https://doi.org/10.1038/ng0996-19
Fuqua WC, Weiner RM (1993) The melA gene is essential for melanin biosynthesis in the marine bacterium Shewanella colwelliana. J Gen Microbiol 139:1105–1114. https://doi.org/10.1099/00221287-139-5-1105
Gonzalez R, Murarka A, Dharmadi Y, Yazdani SS (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10:234–245. https://doi.org/10.1016/j.ymben.2008.05.001
Herrera MC, Krell T, Zhang X, Ramos JL (2009) PhhR binds to target sequences at different distances with respect to RNA polymerase in order to activate transcription. J Mol Biol 394:576–586. https://doi.org/10.1016/j.jmb.2009.09.045
Hunter RC, Newman DK (2010) A putative ABC transporter, HatABCDE, is among molecular determinants of pyomelanin production in Pseudomonas aeruginosa. J Bacteriol 192:5962–5971. https://doi.org/10.1128/JB.01021-10
Keith KE, Killip L, He P, Moran GR, Valvano MA (2007) Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J Bacteriol 189:9057–9065. https://doi.org/10.1128/JB.00436-07
Keller S, Macheleidt J, Scherlach K, Schmaler-Ripcke J, Jacobsen ID, Heinekamp T, Brakhage AA (2011) Pyomelanin formation in Aspergillus fumigatus requires HmgX and the transcriptional activator HmgR but is dispensable for virulence. PLoS One 6:e26604. https://doi.org/10.1371/journal.pone.0026604
Kotob SI, Coon SL, Quintero EJ, Weiner RM (1995) Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a Hyphomonas strain, and Shewanella colwelliana. Appl Environ Microbiol 61:1620–1622
Larionov A, Krause A, Miller W (2005) A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6:62. https://doi.org/10.1186/1471-2105-6-62
Larson TJ, Cantwell JS, van Loo-Bhattacharya AT (1992) Interaction at a distance between multiple operators controls the adjacent, divergently transcribed glpTQ-glpACB operons of Escherichia coli K-12. J Biol Chem 267:6114–6121
Loprasert S, Whangsuk W, Dubbs JM, Sallabhan R, Somsongkul K, Mongkolsuk S (2007) HpdR is a transcriptional activator of Sinorhizobium meliloti hpdA, which encodes a herbicide-targeted 4-hydroxyphenylpyruvate dioxygenase. J Bacteriol 189:3660–3664. https://doi.org/10.1128/JB.01662-06
Martin JP, Batkoff B (1987) Homogentisic acid autoxidation and oxygen radical generation: implications for the etiology of alkaptonuric arthritis. Free Radic Biol Med 3:241–250. https://doi.org/10.1016/S0891-5849(87)80031-X
McFall E, Newman EB (1996) Amino acids as carbon sources. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin EC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella: cellular and molecular biology, 2nd edn. ASM Press, Washington, DC, pp 358–379
Mistry JB, Bukhari M, Taylor AM (2013) Alkaptonuria. Rare Dis 1:e27475. https://doi.org/10.4161/rdis.27475
Morales G, Linares JF, Beloso A, Albar JP, Martínez JL, Rojo F (2004) The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J Bacteriol 186:1337–1344. https://doi.org/10.1128/JB.186.5.1337-1344.2004
Nikel PI, Kim J, de Lorenzo V (2014) Metabolic and regulatory rearrangements underlying glycerol metabolism in Pseudomonas putida KT2440. Environ Microbiol 16:239–254. https://doi.org/10.1111/1462-2920.12224
Noorian P, Jie H, Chen Z, Kjelleberg S, Wilkins MR, Sun S, McDougald D (2017) Pyomelanin produced by Vibrio cholerae confers resistance to predation by Acanthamoeba castellanii. FEMS Microbiol Ecol 93(12). https://doi.org/10.1093/femsec/fix147
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiol 5:203–223. https://doi.org/10.1046/j.1462-5814.2003.00268.x
Palmer GC, Palmer KL, Jorth PA, Whiteley M (2010) Characterization of the Pseudomonas aeruginosa transcriptional response to phenylalanine and tyrosine. J Bacteriol 192:2722–2728. https://doi.org/10.1128/JB.00112-10
Pavan ME, Abbott SL, Zorzópulos J, Janda JM (2000) Aeromonas salmonicida subsp. pectinolytica subsp. nov., a new pectinase-positive subspecies isolated from a heavily polluted river. Int J Syst Evol Microbiol 50:1119–1124. https://doi.org/10.1099/00207713-50-3-1119
Pavan ME, Pavan EE, López NI, Levin L, Pettinari MJ (2015) Living in an extremely polluted environment: clues from the genome of melanin-producing Aeromonas salmonicida subsp. pectinolytica 34melT. Appl Environ Microbiol 81:5235–5248. https://doi.org/10.1128/AEM.00903-15
Plonka PM, Grabacka M (2006) Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim Pol 53:429–443
Rocaboy-Faquet E, Noguer T, Romdhane S, Bertrand C, Dayan FE, Barthelmebs L (2014) Novel bacterial bioassay for a high-throughput screening of 4-hydroxyphenylpyruvate dioxygenase inhibitors. Appl Microbiol Biotechnol 98:7243–7252. https://doi.org/10.1007/s00253-014-5793-5
Rodríguez-Rojas A, Mena A, Martín S, Borrell N, Oliver A, Blázquez J (2009) Inactivation of the hmgA gene of Pseudomonas aeruginosa leads to pyomelanin hyperproduction, stress resistance and increased persistence in chronic lung infection. Microbiology 155:1050–1057. https://doi.org/10.1099/mic.0.024745-0
Rojo F (2010) Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev 34:658–684. https://doi.org/10.1111/j.1574-6976.2010.00218.x
Ryan A, Kaplan E, Nebel JC, Polycarpou E, Crescente V, Lowe E, Preston GM, Sim E (2014) Identification of NAD(P)H quinone oxidoreductase activity in azoreductases from P. aeruginosa: azoreductases and NAD(P)H quinone oxidoreductases belong to the same FMN-dependent superfamily of enzymes. PLoS One 9:e98551. https://doi.org/10.1371/journal.pone.0098551
Sanchez-Amat A, Ruzafa C, Solano F (1998) Comparative tyrosine degradation in Vibrio cholerae strains. The strain ATCC 14035 as a prokaryotic melanogenic model of homogentisate-releasing cell. Comp Biochem Physiol B Biochem Mol Biol 119:557–562. https://doi.org/10.1016/S0305-0491(98)00028-5
Santucci A, Bernardini G, Braconi D, Petricci E, Manetti F (2017) 4-Hydroxyphenylpyruvate dioxygenase and its inhibition in plants and animals: small molecules as herbicides and agents for the treatment of human inherited diseases. J Med Chem 60:4101–4125. https://doi.org/10.1021/acs.jmedchem.6b01395
Solano F (2014) Melanins: skin pigments and much more - types, structural models, biological functions, and formation routes. New J Sci 1:1–28. https://doi.org/10.1155/2014/498276
Stepanova V, Rodionov DA (2011) Genomic analysis of transcriptional regulation of aromatic amino acid metabolism in gamma-proteobacteria. Department of Bioengineering and Bioinformatics of MV Lomonosov Moscow State University 352:186–188
Stuber K, Burr SE, Braun M, Wahli T, Frey J (2003) Type III secretion genes in Aeromonas salmonicida subsp salmonicida are located on a large thermolabile virulence plasmid. J Clin Microbiol 41:3854–3856. https://doi.org/10.1128/JCM.41.8.3854-3856.2003
Tanaka KH, Vincent AT, Emond-Rheault JG, Adamczuk M, Frenette M, Charette SJ (2017) Plasmid composition in Aeromonas salmonicida subsp. salmonicida 01-B526 unravels unsuspected type three secretion system loss patterns. BMC Genomics 18:528. https://doi.org/10.1186/s12864-017-3921-1
Turick CE, Caccavo F Jr, Tisa LS (2003) Electron transfer from Shewanella algae BrY to hydrous ferric oxide is mediated by cell-associated melanin. FEMS Microbiol Lett 220:99–104. https://doi.org/10.1016/S0378-1097(03)00096-X
Turick CE, Knox AS, Becnel JM, Ekechukwu AA, Milliken CE (2010) Properties and function of pyomelanin. In: Elnashar MM (ed) Biopolymers, 1st edn. Sciyo, Rijeka, pp 449–472. https://doi.org/10.5772/10273
Upton C, Buckley JT (1995) A new family of lipolytic enzymes? Trends Biochem Sci 20:178–179. https://doi.org/10.1016/S0968-0004(00)89002-7
Vanden Bergh P, Heller M, Braga-Lagache S, Frey J (2013) The Aeromonas salmonicida subsp. salmonicida exoproteome: determination of the complete repertoire of Type-Three Secretion System effectors and identification of other virulence factors. Proteome Sci 11:42. https://doi.org/10.1186/1477-5956-11-42
Vincent AT, Rouleau FD, Moineau S, Charette SJ (2017) Study of mesophilic Aeromonas salmonicida A527 strain sheds light on the species’ lifestyles and taxonomic dilemma. FEMS Microbiol Lett 364(23). https://doi.org/10.1093/femsle/fnx239
Wang H, Qiao Y, Chai B, Qiu C, Chen X (2015) Identification and molecular characterization of the homogentisate pathway responsible for pyomelanin production, the major melanin constituents in Aeromonas media WS. PLoS One 10:e0120923. https://doi.org/10.1371/journal.pone.0120923
Wu L, Lin X, Peng X (2009) From proteome to genome for functional characterization of pH-dependent outer membrane proteins in Escherichia coli. J Proteome Res 8:1059–1070. https://doi.org/10.1021/pr800818r
Yu HB, Zhang YL, Lau YL, Yao F, Vilches S, Merino S, Tomas JM, Howard SP, Leung KY (2005) Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Appl Environ Microbiol 71:4469–4477. https://doi.org/10.1128/AEM.71.8.4469-4477.2005
Zatkova A (2011) An update on molecular genetics of Alkaptonuria (AKU). J Inherit Metab Dis 34:1127–1136. https://doi.org/10.1007/s10545-011-9363-z
Zeng Z, Guo XP, Cai X, Wang P, Li B, Yang JL, Wang X (2017) Pyomelanin from Pseudoalteromonas lipolytica reduces biofouling. Microb Biotechnol 10:1718–1731. https://doi.org/10.1111/1751-7915.12773
Acknowledgments
N.I.L. and M.J.P. are career investigators from CONICET. E.S.V. and D.E.E. hold doctoral fellowships from CONICET.
Funding
This work was partially supported by the University of Buenos Aires, CONICET, and Agencia Nacional de Promoción Científica y Tecnológica.
Author information
Authors and Affiliations
Contributions
M.E.P., N.I.L., and M.J.P. conceived the project and designed experiments. All authors carried out experiments. M.E.P., N.I.L., and M.J.P. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 213 kb)
Rights and permissions
About this article
Cite this article
Pavan, M.E., Venero, E.S., Egoburo, D.E. et al. Glycerol inhibition of melanin biosynthesis in the environmental Aeromonas salmonicida 34melT. Appl Microbiol Biotechnol 103, 1865–1876 (2019). https://doi.org/10.1007/s00253-018-9545-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9545-9