Abstract
Purpose
The liver is perfused through the portal vein and the hepatic artery. When its perfusion is assessed using positron emission tomography (PET) and 15O-labeled water (H2 15O), calculations require a dual blood input function (DIF), i.e., arterial and portal blood activity curves. The former can be generally obtained invasively, but blood withdrawal from the portal vein is not feasible in humans. The aim of the present study was to develop a new technique to estimate quantitative liver perfusion from H2 15O PET images with a completely non-invasive approach.
Methods
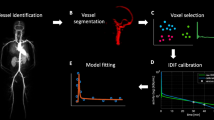
We studied normal pigs (n = 14) in which arterial and portal blood tracer concentrations and Doppler ultrasonography flow rates were determined invasively to serve as reference measurements. Our technique consisted of using model DIF to create tissue model function and the latter method to simultaneously fit multiple liver time–activity curves from images. The parameters obtained reproduced the DIF. Simulation studies were performed to examine the magnitude of potential biases in the flow values and to optimize the extraction of multiple tissue curves from the image.
Results
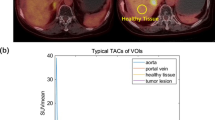
The simulation showed that the error associated with assumed parameters was <10%, and the optimal number of tissue curves was between 10 and 20. The estimated DIFs were well reproduced against the measured ones. In addition, the calculated liver perfusion values were not different between the methods and showed a tight correlation (r = 0.90).
Conclusion
In conclusion, our results demonstrate that DIF can be estimated directly from tissue curves obtained through H2 15O PET imaging. This suggests the possibility to enable completely non-invasive technique to assess liver perfusion in patho-physiological studies.










Similar content being viewed by others
References
Alenius S, Ruotsalainen U. Bayesian image reconstruction for emission tomography based on median root prior. Eur J Nucl Med. 1997;24:258–65.
Becker GA, Muller-Schauenburg W, Spilker ME, Machulla HJ, Piert M. A priori identifiability of a one-compartment model with two input functions for liver blood flow measurements. Phys Med Biol. 2005;50:1393–404.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Blomley MJ, Coulden R, Dawson P, et al. Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr. 1995;19:424–33.
Carson RE. Parameter estimation in positron emission tomography. In: Phelps ME, Mazziotta JC, Schelbert HR, editors. Positron emission tomography and autoradiography: principles and applications for the brain and heart. New York, NY: Raven; 1986. p. 347–90.
Johnson DJ, Muhlbacher F, Wilmore DW. Measurement of hepatic blood flow. J Surg Res. 1985;39:470–81.
Edward VR, Di Bella EV, Clackdoyle R, Gullberg GT. Blind estimation of compartmental model parameters. Phys Med Biol. 1999;44:765–80.
Eriksson L, Holte S, Bohm Chr, Kesselberg M, Hovander B. Automated blood sampling system for positron emission tomography. IEEE Trans Nucl Sci. 1988;35:703–7.
Eriksson L, Kanno I. Blood sampling devices and measurements. Med Prog Technol. 1991;17:249–57.
Henderson JM, Gilmore GT, Mackay GJ, Galloway JR, Dodson TF, Kutner MH. Hemodynamics during liver transplantation: the interactions between cardiac output and portal venous and hepatic arterial flows. Hepatology. 1992;16:715–8.
Iida H, Kanno I, Miura S, Murakami M, Takahashi K, Uemura K. Error analysis of a quantitative cerebral blood flow measurement using H2 15O autoradiography and positron emission tomography, with respect to the dispersion of the input function. J Cereb Blood Flow Metab. 1986;6:536–45.
Iida H, Higano S, Tomura N, Shishido F, Kanno I, Miura S, et al. Evaluation of regional differences of tracer appearance time in cerebral tissues using [15O] water and dynamic positron emission tomography. J Cereb Blood Flow Metab. 1988;8:285–8.
Iozzo P, Gastaldelli A, Järvisalo MJ, Kiss J, Borra R, Buzzigoli E, et al. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: a validation study. J Nucl Med. 2006;47:1016–22.
Iozzo P, Järvisalo MJ, Kiss J, Borra R, Naum GA, Viljanen A, et al. Quantification of liver glucose metabolism by positron emission tomography: validation study in pigs. Gastroenterology. 2007;132:531–42.
Juillard L, Janier M, Fouque D, et al. Renal blood flow measurement by positron emission tomography using 15O-labeled water. Kidney Int. 2000;57:2511–8.
Kanno I, Iida H, Miura S, Murakami M, Takahashi K, Sasaki H, et al. A system for cerebral blood flow measurement using an H2 15O autoradiographic method and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:143–53.
Kudomi N, Choi E, Watabe H, Kim KM, Shidahara M, Ogawa M, et al. Development of a GSO detector assembly for a continuous blood sampling system. IEEE TNS. 2003;50:70–3.
Kudomi N, Watabe H, Hayashi T, Iida H. Non-invasive estimation of arterial input function for water and oxygen from PET dynamic images. J Nucl Med. 2006;47(Supplement 1):361.
Leen E, Goldberg JA, Anderson JR, et al. Hepatic perfusion changes in patients with liver metastases: comparison with those patients with cirrhosis. Gut. 1993;34:554–7.
Lindstedt, Schaeffer. Use of allometry in predicting anatomical and physiological parameters of mammals. Laboratory Anim. 2002;36:1–19.
Martin-Comin J, Mora J, Figueras J, et al. Calculation of portal contribution to hepatic blood flow with 99m-Tc-microcolloids. A noninvasive method to diagnose liver graft rejection. J Nucl Med. 1988;29:1776–80.
Materne R, Van Beers BE, Smith AM, Leconte I, Jamart J, Dehoux JP, et al. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond). 2000;99:517–25.
Munk OL, Bass L, Roelsgaard K, Bender D, Hansen SB, Keiding S. Liver kinetics of glucose analogs measured in pigs by PET: importance of dual-input blood sampling. Nucl Med. 2001;42:795–801.
Naganawa M, Kimura Y, Nariai T, et al. Omission of serial arterial blood sampling in neuroreceptor imaging with independent component analysis. NeuroImage. 2005a;26:885–90.
Naganawa M, Kimura Y, Ishii K, Oda K, Ishiwata K, Matani A. Extraction of a plasma time–activity curve from dynamic brain pet images based on independent component analysis. IEEE Trans on Bio-Med Eng. 2005b;52:201–10.
Ruotsalainen U, Raitakari M, Nuutila P, Oikonen V, Sipilä H, Teräs M, et al. Quantitative blood flow measurement of skeletal muscle using oxygen-15-water and PET. J Nucl Med. 1997;38:314–9.
Sanabria-Bohorquez SM, Maes A, Dupont P, Bormans G, de Groot T, Coimbra A, et al. Image-derived input function for [11C]flumazenil kinetic analysis in human brain. Mol Img Biol. 2003;5:72–8.
Taniguchi H, Oguro A, Takeuchi K, Miyata K, Takahashi T, Inaba T, et al. Difference in regional hepatic blood flow in liver segments—non-invasive measurement of regional hepatic arterial and portal blood flow in human by positron emission tomography with H2(15)O. Ann Nucl Med. 1993;7:141–5.
Taniguchi H, Oguro A, Koyama H, Masuyama M, Takahashi T. Analysis of models for quantification of arterial and portal blood flow in the human liver using PET. J Comput Assist Tomogr. 1996a;20:135–44.
Taniguchi H, Koyama H, Masuyama M, Takada A, Mugitani T, Tanaka H, et al. Angiotensin-II-induced hypertension chemotherapy: evaluation of hepatic blood flow with oxygen-15 PET. J Nucl Med. 1996b;37:1522–3.
Taniguchi H, Yamaguchi A, Kunishima S, Koh T, Masuyama M, Koyama H, et al. Using the spleen for time-delay correction of the input function in measuring hepatic blood flow with oxygen-15 water by dynamic PET. Ann Nucl Med. 1999;13:215–21.
Van der Weerdt A, Klein LJ, Boellaard R, Visser CA, Visser FC, Lammertsma AA. Image-derived input functions for determination of MRGlu in cardiac 18F-FDG PET scans. J Nucl Med. 2001;42:1622–9.
Votaw JR, Shulman SD. Performance evaluation of the pico-count flow-through detector for use in cerebral blood flow PET studies. J Nucl Med. 1998;39:509–15.
Watabe H, Channing MA, Riddell C, Jousse F, Libutti SK, Carrasquillo JA, et al. Noninvasive estimation of the aorta input function for measurement of tumor blood flow with. IEEE Trans Med Imaging. 2001;20:164–74.
Ziegler SI, Haberkorn U, Byrne H, Tong C, Kaja S, Richolt JA, et al. Price P Measurement of liver blood flow using oxygen-15 labelled water and dynamic positron emission tomography: limitations of model description. Eur J Nucl Med. 1996;23:169–77.
Acknowledgments
The authors thank the technical staff of the Turku PET Centre for the efforts and skills dedicated to this project. This work is part of the project Hepatic and Adipose Tissue and Functions in the Metabolic Syndrome (HEPADIP, see http://www.hepadip.org/), which is supported by the European Commission as an Integrated Project under the 6th Framework Programme (contract LSHMCT-2005-018734). The study was further supported by grants from the Academy of Finland (206359 to P.N.), Finnish Diabetes Foundation (P.I.), EFSD/Eli-Lilly (P.I.), Sigrid Juselius Foundation (N.K. and P.I.), and Novo Nordisk Foundation (P.N.).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
A model function for AIF was created by assuming a two-compartment model in which the tracer is administered in a rectangular form and diffuses bi-directionally between arterial and interstitial space in whole body peripheral tissue compartments. Differential equations for the model function (C A(t)) can be expressed as
where t 1 and t 2 assumes the appearance time of administered tracer, and t 2 − t 1 represents the administration duration; A corresponds to the given amount of tracer. The equation F (Eq. 11) represents the bolus administration of tracer in the rectangular form with duration t 2 − t 1. C WB(t) is the expected tracer concentration in interstitial spaces in whole body peripheral tissues; K e and K i are bidirectional tracer diffusion rates between blood and peripheral tissue compartments, respectively. Solving Eq. 10 for C WB gives
Thus,
Substitution of C WB from Eq. 12 into 14 after multiplying \(e^{K_{\text{i}} \cdot t} \) gives
Differentiation with respect to t after arranging gives
where α = K i/K e . Thus,
Solving Eq. 17, we obtain
The first term in the second equation for t 1 < t <t 2, i.e., K e α(1 + α)(t 1 − t 2), would complicate further calculations (such as tissue response and portal input); thus, this term was omitted, and the model function (Eq. 18) was modified to set the C A value as 0 at t = t 1, as continuous at t = t 2, and as non-zero value at the equilibrium, i.e., at t = ∞. Thus, the following equation was derived:
Rights and permissions
About this article
Cite this article
Kudomi, N., Slimani, L., Järvisalo, M.J. et al. Non-invasive estimation of hepatic blood perfusion from H2 15O PET images using tissue-derived arterial and portal input functions. Eur J Nucl Med Mol Imaging 35, 1899–1911 (2008). https://doi.org/10.1007/s00259-008-0796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0796-z