Abstract
Purpose
The biokinetics and dosimetry of 111In-DOTA-NOC-ATE (NOCATE), a high-affinity ligand of SSTR-2 and SSTR-5, and 111In-DTPA-octreotide (Octreoscan™, OCTREO) were compared in the same patients.
Methods
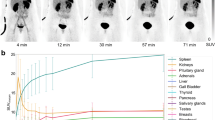
Seventeen patients (10 men, 7 women; mean age 60 years), referred for an OCTREO scan for imaging of a neuroendocrine tumour (15), thymoma (1) or medullary thyroid carcinoma (1), agreed to undergo a second study with NOCATE. Whole-body anterior–posterior scans were recorded 0.5 (100 % reference scan), 4, 24 and 48 h (17 patients) and 120 h (5 patients) after injection. In 16 patients the OCTREO scan (178 ± 15 MBq) was performed 16 ± 5 days before the NOCATE scan (108 ± 14 MBq) with identical timing; 1 patient had the NOCATE scan before the OCTREO scan. Blood samples were obtained from 14 patients 5 min to 48 h after injection. Activities expressed as percent of the initial (reference) activity in the whole body, lung, kidney, liver, spleen and blood were fitted to biexponential or single exponential functions. Dosimetry was performed using OLINDA/EXM.
Results
Initial whole-body, lung and kidney activities were similar, but retention of NOCATE was higher than that of OCTREO. Liver and spleen uptakes of NOCATE were higher from the start (p < 0.001) and remained so over time. Whole-body activity showed similar α and β half-lives, but the β fraction of NOCATE was double that of OCTREO. Blood T 1/2β for NOCATE was longer (19 vs. 6 h). As a result, the effective dose of NOCATE (105 μSv/MBq) exceeded that of OCTREO (52 μSv/MBq), and the latter result was similar to the ICRP 106 value of 54 μSv/MBq. Differential activity measurement in blood cells and plasma showed an average of <5 % of NOCATE and OCTREO attached to globular blood components.
Conclusion
NOCATE showed a slower clearance from normal tissues and its effective dose was roughly double that of OCTREO.





Similar content being viewed by others
References
Reubi JC, Perrin MH, Rivier JE, Vale W. High affinity binding sites for a somatostatin-28 analog in rat brain. Life Sci. 1981;28:2191–8.
Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98.
Ginj M, Chen J, Reubi JC, Maecke HR. Preclinical evaluation of new and highly potent analogs of octreotide for targeted radiotherapy. Eur J Nucl Med Mol Imaging. 2003;30:S198–9.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–46.
Ginj M, Chen J, Walter MA, Eltschinger V, Reubi JC, Maecke HR. Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res. 2005;11:1136–45.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Wessels BW, Bolch WE, Bouchet LG, Breitz HB, Denardo GL, Meredith RF, et al. Bone marrow dosimetry using blood-based models for radiolabeled antibody therapy: a multiinstitutional comparison. J Nucl Med. 2004;45:1725–33.
Meyer SL. Data analysis for scientists and engineers. New York: Wiley; 1975. ISBN: 0-471-59995-6.
ICRP. Radiation dose to patients from radiopharmaceuticals – addendum 3 to ICRP publication 53. ICRP publication 106. Ann ICRP. 38(1-2)
Stabin MG, Kooij PP, Bakker WH, Inoue T, Endo K, Coveney J, et al. Radiation dosimetry for indium-111-pentetreotide. J Nucl Med. 1997;38:1919–22.
Pettinato C, Sarnelli A, Di Donna M, Civollani S, Nanni C, Montini G, et al. 68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2008;35:72–9.
Acknowledgments
The support of the National Foundation for Scientific Research is gratefully acknowledged. The clinical study was performed upon the commitment of the departments of nuclear medicine of the Lausanne and Geneva University Hospitals.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boubaker, A., Prior, J.O., Willi, JP. et al. Biokinetics and dosimetry of 111In-DOTA-NOC-ATE compared with 111In-DTPA-octreotide. Eur J Nucl Med Mol Imaging 39, 1868–1875 (2012). https://doi.org/10.1007/s00259-012-2210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2210-0