Abstract
Purpose
The aim of this study was to investigate MRI-derived diffusion weighted imaging (DWI) and arterial spin labeling (ASL) perfusion imaging in comparison with 18F–dihydroxyphenylalanine (DOPA) PET with respect to diagnostic performance in tumor grading and outcome prediction in pediatric patients with diffuse astrocytic tumors (DAT).
Methods
We retrospectively analyzed 26 children with histologically proven treatment naïve low and high grade DAT who underwent ASL and DWI performed within 2 weeks of 18F–DOPA PET. Relative ASL-derived cerebral blood flow max (rCBF max) and DWI-derived minimum apparent diffusion coefficient (rADC min) were compared with 18F–DOPA uptake tumor/normal tissue (T/N) and tumor/striatum (T/S) ratios, and correlated with World Health Organization (WHO) tumor grade and progression-free survival (PFS). Statistics included Pearson’s chi-square and Mann-Whitney U tests, Spearman’s rank correlation, receiver operating characteristic (ROC) analysis, discriminant function analysis (DFA), Kaplan-Meier survival curve, and Cox analysis.
Results
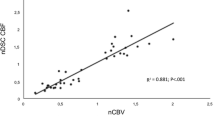
A significant correlation was demonstrated between rCBF max, rADC min, and 18F–DOPA PET data (p < 0.001). Significant differences in terms of rCBF max, rADC min, and 18F–DOPA uptake were found between low- and high-grade DAT (p ≤ 0.001). ROC analysis and DFA demonstrated that T/S and T/N values were the best parameters for predicting tumor progression (AUC 0.93, p < 0.001). On univariate analysis, all diagnostic tools correlated with PFS (p ≤ 0.001); however, on multivariate analysis, only 18F–DOPA uptake remained significantly associated with outcome (p ≤ 0.03), while a trend emerged for rCBF max (p = 0.09) and rADC min (p = 0.08). The combination of MRI and PET data increased the predictive power for prognosticating tumor progression (AUC 0.97, p < 0.001).
Conclusions
DWI, ASL and 18F–DOPA PET provide useful complementary information for pediatric DAT grading. 18F–DOPA uptake better correlates with PFS prediction. Combining MRI and PET data provides the highest predictive power for prognosticating tumor progression suggesting a synergistic role of these diagnostic tools.


Similar content being viewed by others
References
Vossough A, Nabavizadeh SA. Functional imaging based diagnostic strategy: intra-axial brain masses. In: Scott HF, Feroze BM, editors. Functional Neuroradiology, principles and clinical application. New York: Springer; 2011. p. 197–220.
Mabray MC, Barajas RF Jr, Cha S. Modern brain tumor imaging. Brain Tumor Res Treat. 2015;3:8–23.
Rossi A, Gandolfo C, Morana G, Severino M, Garrè ML, Cama A. New MR sequences (diffusion, perfusion, spectroscopy) in brain tumours. Pediatr Radiol. 2010;40:999–1009.
Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology. 2003;228:523–32.
Wolf RL, Wang J, Wang S, Melhem ER, O'Rourke DM, Judy KD, et al. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging. 2005;22:475–82.
Ata ES, Turgut M, Eraslan C, Dayanır YÖ. Comparison between dynamic susceptibility contrast magnetic resonance imaging and arterial spin labeling techniques in distinguishing malignant from benign brain tumors. Eur J Radiol. 2016;85:1545–53.
Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, et al. Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol. 2014;35:395–401.
Dangouloff-Ros V, Grevent D, Pagès M, Blauwblomme T, Calmon R, Elie C, et al. Choroid plexus Neoplasms: toward a distinction between carcinoma and Papilloma using arterial spin-Labeling. AJNR Am J Neuroradiol. 2015;36:1786–90.
Nabavizadeh SA, Assadsangabi R, Hajmomenian M, Santi M, Vossough A. High accuracy of arterial spin labeling perfusion imaging in differentiation of pilomyxoid from pilocytic astrocytoma. Neuroradiol. 2015;57:527–33.
Dangouloff-Ros V, Deroulers C, Foissac F, Badoual M, Shotar E, Grévent D, et al. Arterial spin Labeling to predict brain tumor grading in children: correlations between Histopathologic vascular density and perfusion MR imaging. Radiology. 2016;281:553–66.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response assessment in Neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncol. 2016;18:1199–208.
Heiss WD, Raab P, Lanfermann H. Multimodality assessment of brain tumors and tumor recurrence. J Nucl Med. 2011;52:1585–600.
Morana G, Piccardo A, Milanaccio C, Puntoni M, Nozza P, Cama A, et al. Value of 18F-3,4-dihydroxyphenylalanine PET/MR image fusion in pediatric supratentorial infiltrative astrocytomas: a prospective pilot study. J Nucl Med. 2014;55:718–23.
Morana G, Piccardo A, Puntoni M, Nozza P, Cama A, Raso A, et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro-Oncol. 2015;17:1637–47.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of Tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72.
van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–93.
Sorensen AG, Patel S, Harmath C, Bridges S, Synnott J, Sievers A, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19:551–7.
Morana G, Piccardo A, Garrè ML, Cabria M, Rossi A. 18F-DOPA uptake of developmental venous anomalies in children with brain Tumors. Clin Nucl Med. 2016;41:e351–2.
Berntsson SG, Falk A, Savitcheva I, Godau A, Zetterling M, Hesselager G, et al. Perfusion and diffusion MRI combined with 11C-methionine PET in the preoperative evaluation of suspected adult low-grade gliomas. J Neuro-Oncol. 2013;11:241–9.
Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55:1611–6.
Caulo M, Panara V, Tortora D, Mattei PA, Briganti C, Pravatà E, et al. Data-driven grading of brain gliomas: a multiparametric MR imaging study. Radiology. 2014;272:494–503.
Morana G, Piccardo A, Garrè ML, Nozza P, Consales A, Rossi A. Multimodal magnetic resonance imaging and 18F-L-dihydroxyphenylalanine positron emission tomography in early characterization of pseudoresponse and nonenhancing tumor progression in a pediatric patient with malignant transformation of ganglioglioma treated with bevacizumab. J Clin Oncol. 2013;31:e1–5.
Hipp SJ, Steffen-Smith EA, Patronas N, Herscovitch P, Solomon JM, Bent RS, et al. Molecular imaging of pediatric brain tumors: comparison of tumor metabolism using 18F FDG-PET and MRSI. J Neuro-Oncol. 2012;109:521–7.
Fraioli F, Shankar A, Hargrave D, Hyare H, Gaze MN, Groves AM, et al. 18F-fluoroethylcholine (18F-Cho) PET/MRI functional parameters in pediatric astrocytic brain tumors. Clin Nucl Med. 2015;40:e40–5.
Zukotynski KA, Fahey FH, Vajapeyam S, Ng SS, Kocak M, Gururangan S, et al. Exploratory evaluation of MR permeability with 18F-FDG PET mapping in Pediatric brain Tumors: a report from the Pediatric brain tumor consortium. J Nucl Med. 2013;54:1237–43.
Zukotynski K, Vajapeyam S, Fahey FH, Kocak M, Brown D, Ricci K, et al. Correlation of 18F-FDG PET and MR apparent diffusion coefficient (ADC) histogram metrics with survival in diffuse intrinsic Pontine Glioma: a report from the Pediatric brain tumor consortium. J Nucl Med. 2017; doi:10.2967/jnumed.116.185389.
Fink JR, Muzi M, Peck M, Krohn KA. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J Nucl Med. 2015;56:1554–61.
Werner P, Barthel H, Drzezga A, Sabri O. Current status and future role of brain PET/MRI in clinical and research settings. Eur J Nucl Med Mol Imaging. 2015;42:512–26.
Henriksen OM, Larsen VA, Muhic A, Hansen AE, Larsson HB, Poulsen HS, et al. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [(18)F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur J Nucl Med Mol Imaging. 2016;43:103–12.
Jones DT, Mulholland SA, Pearson DM, Malley DS, Openshaw SW, Lambert SR, et al. Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol. 2011;121:753–61.
Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28:3061–8.
Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain Tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015;33:2986–98.
Huisman TA. Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur Radiol. 2003;13:2283–97.
Kan P, Liu JK, Hedlund G, Brockmeyer DL, Walker ML, Kestle JR. The role of diffusion-weighted magnetic resonance imaging in pediatric brain tumors. Childs Nerv Syst. 2006;22:1435–9.
Lequin M, Hendrikse J. Advanced MR imaging in Pediatric brain Tumors, clinical applications. Neuroimaging Clin N Am. 2017;27:167–90.
Lober RM, Cho YJ, Tang Y, Barnes PD, Edwards MS, Vogel H, et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neuro-Oncol. 2014;117:175–82.
Rose S, Fay M, Thomas P, Bourgeat P, Dowson N, Salvado O, et al. Correlation of MRI-derived apparent diffusion coefficients in newly diagnosed gliomas with [18F]-fluoro-L-dopa PET: what are we really measuring with minimum ADC? AJNR Am J Neuroradiol. 2013;34:758–64.
Karavaeva E, Harris RJ, Leu K, Shabihkhani M, Yong WH, Pope WB, et al. Relationship between [18F]FDOPA PET uptake, apparent diffusion coefficient (ADC), and proliferation rate in recurrent malignant Gliomas. Mol Imaging Biol. 2015;17:434–42.
Morana G, Puntoni M, Garrè ML, Massollo M, Lopci E, Naseri M, et al. Ability of (18)F-DOPA PET/CT and fused (18)F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur J Nucl Med Mol Imaging. 2016;43:1664–72.
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–16.
Acknowledgments
This work was supported, in part, by the Associazione per la ricerca sui tumori cerebrali del bambino (ARTUCEBA) and Fondazione Guido Berlucchi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
For this type of study (retrospective study) formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Informed consent was signed from all patients or their legal guardians, and patient assent was obtained whenever appropriate.
Electronic supplementary material
ESM 1
(DOCX 105 kb).
Rights and permissions
About this article
Cite this article
Morana, G., Piccardo, A., Tortora, D. et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F–DOPA PET. Eur J Nucl Med Mol Imaging 44, 2084–2093 (2017). https://doi.org/10.1007/s00259-017-3777-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3777-2