Abstract
Introduction
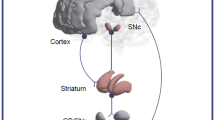
This study aims to reveal the feasibility and potential of molecular connectivity based on neurotransmission in comparison with the metabolic connectivity with an application to dopaminergic pathways. For this purpose, we propose to compare the neurotransmission connectivity findings using 123I-FP-CIT SPECT and 18F-FDOPA PET with the metabolic connectivity findings using 18F-FDG PET.
Methods
18F-FDG PET and 123I-FP-CIT SPECT images from 47 subjects and 18F-FDOPA PET images from 177 subjects, who had no neurological or psychiatric disorders, were studied. Interregional correlation analyses were performed at the group level to determine the midbrain’s connectivity via glucose metabolic rate using 18F-FDG PET and via dopaminergic binding potential using 123I-FP-CIT SPECT and 18F-FDOPA PET. SPM-T maps of each radiotracer were generated, and masks used to highlight the significant differences obtained among the imaging modalities and targets.
Results
The three dopaminergic pathways (i.e., nigrostriatal, mesolimbic, and mesocortical) were identified by 18F-FDG PET (1599 voxels, with a Tmax value of 12.6), 123I-FP-CIT SPECT (1120 voxels, with Tmax value of 5.1), and 18F-FDOPA PET (6054 voxels, with Tmax value of 11.7) for a T voxel threshold of 5.10, 2.80, and 5.10, respectively. Using the same T voxel threshold of 5.10, 18F-FDOPA PET showed more specific findings than 18F-FDG PET with less voxels identified outside these pathways (− 9323 voxels), whereas no significant voxels were obtained with 123I-FP-CIT SPECT at this threshold.
Conclusion
The present study illustrates the feasibility and interest in using molecular connectivity with 18F-FDOPA PET for dopaminergic pathways. Such analyses could be applied to specific diseases involving the dopaminergic system.





Similar content being viewed by others
References
Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1984;4:484–99.
Metter EJ, Riege WH, Kameyama M, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in Alzheimer’s, Huntington’s, and Parkinson’s diseases. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1984;4:500–6.
Clark CM, Stoessl AJ. Glucose use correlations: a matter of inference. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1986;6:511–2.
Yakushev I, Drzezga A, Habeck C. Metabolic connectivity: methods and applications. Curr Opin Neurol. 2017;30:677–85.
Verger A, Klesse E, Chawki MB, Witjas T, Azulay J-P, Eusebio A, et al. Brain PET substrate of impulse control disorders in Parkinson’s disease: a metabolic connectivity study. Hum Brain Mapp. 2018;39:3178–86.
The REMPET Study Group Sittig-Wiegand Elisabeth f Depboylu Candan f Reetz Kathrin l Overeem Sebastiaan m Pijpers Angelique m Reesink Fransje E. b van Laar Teus b Teune Laura K. b Höffken Helmut n Luster Marcus n Timmermann Lars f Kesper Karl o Adriaanse Sofie M. p Booij Jan p Sambuceti Gianmario d Girtler Nicola a, Arnaldi D, Meles SK, Giuliani A, Morbelli S, Renken RJ, et al. Brain glucose metabolism heterogeneity in idiopathic REM Sleep behavior disorder and in parkinson’s disease. J Park Dis. 2019;9:229–39.
Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, et al. Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging. 2008;35:1681–91.
Morbelli S, Drzezga A, Perneczky R, Frisoni GB, Caroli A, van Berckel BNM, et al. Resting metabolic connectivity in prodromal Alzheimer’s disease. A European Alzheimer Disease Consortium (EADC) project. Neurobiol Aging. 2012;33:2533–50.
Gardner A, Åstrand D, Öberg J, Jacobsson H, Jonsson C, Larsson S, et al. Towards mapping the brain connectome in depression: functional connectivity by perfusion SPECT. Psychiatry Res Neuroimaging. 2014;223:171–7.
Hahn A, Lanzenberger R, Kasper S. Making Sense of Connectivity. Int J Neuropsychopharmacol. 2019;22:194–207.
Tuominen L, Nummenmaa L, Keltikangas-Järvinen L, Raitakari O, Hietala J. Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems: mapping neurotransmitter networks with PET. Hum Brain Mapp. 2014;35:1875–84.
Vanicek T, Kutzelnigg A, Philippe C, Sigurdardottir HL, James GM, Hahn A, et al. Altered interregional molecular associations of the serotonin transporter in attention deficit/hyperactivity disorder assessed with PET. Hum Brain Mapp. 2017;38:792–802.
Veronese M, Moro L, Arcolin M, Dipasquale O, Rizzo G, Expert P, et al. Covariance statistics and network analysis of brain PET imaging studies. Sci Rep. 2019;9:2496.
Carlsson A, Lindqvist M, Magnusson T, Waldeck B. On the presence of 3-hydroxytyramine in brain. Science. 1958;127:471.
Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41:333–40.
Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl). 2006;188:567–85.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35:4–26.
Fuxe K, Agnati LF, Kalia M, Goldstein M, Andersson K, Härfstrand A. Dopaminergic systems in the brain and pituitary. Dopaminergic Syst [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 1985 [cited 2019 Apr 8]. p. 11–25. Available from: http://www.springerlink.com/index/10.1007/978-3-642-69948-1_2 SMASH
Bloom FE, Björklund A, Hökfelt T. The primate nervous system. Part I. Amsterdam. New York: Elsevier; 1997.
Dunnett SB. Dopamine [Internet]. Amsterdam; Boston: Elsevier; 2005 [cited 2019 Apr 8]. Available from: http://www.sciencedirect.com/science/book/9780444517784
Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci 1962. 1964;3:523–30.
Andén N-E, Dahlström A, Fuxe K, Larsson K. Further evidence for the presence of nigro-neostriatal dopamine neurons in the rat: nigro-neostriatal dopamine neurons. Am J Anat. 1965;116:329–33.
Hu Z, Cooper M, Crockett DP, Zhou R. Differentiation of the midbrain dopaminergic pathways during mouse development. J Comp Neurol. 2004;476:301–11.
Gerfen CR. Functional neuroanatomy of dopamine in the striatum. In: Iversen L, Iversen S, Dunnett S, Bjorklund A, editors. Dopamine Handb [Internet]. Oxford University Press; 2009 [cited 2019 Apr 8]. p. 11–21. Available from: http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780195373035.001.0001/acprof-9780195373035-chapter-2 SMASH
Nestler EJ, Hyman SE, Malenka RC. Molecular neuropharmacology: a foundation for clinical neuroscience. 2nd ed. New York: McGraw-Hill Medical; 2009.
Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–63.
Eusebio A, Azulay J-P, Ceccaldi M, Girard N, Mundler O, Guedj E. Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur J Nucl Med Mol Imaging. 2012;39:1778–83.
Darcourt J, Schiazza A, Sapin N, Dufour M, Ouvrier MJ, Benisvy D, et al. 18F-FDOPA PET for the diagnosis of parkinsonian syndromes. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Of. 2014;58:355–65.
Toch S-R, Poussier S, Micard E, Bertaux M, Van Der Gucht A, Chevalier E, et al. Physiological whole-brain distribution of [18F]FDOPA uptake index in relation to age and gender: results from a voxel-based semi-quantitative analysis. Mol Imaging Biol [Internet]. 2018 [cited 2019 Mar 11]; Available from: http://link.springer.com/10.1007/s11307-018-1256-1 SMASH
Hoffman JM, Melega WP, Hawk TC, Grafton SC, Luxen A, Mahoney DK, et al. The effects of carbidopa administration on 6-[18F]fluoro-L-dopa kinetics in positron emission tomography. J Nucl Med Off Publ Soc Nucl Med. 1992;33:1472–7.
Verger A, Roman S, Chaudat R-M, Felician O, Ceccaldi M, Didic M, et al. Changes of metabolism and functional connectivity in late-onset deafness: evidence from cerebral 18F-FDG-PET. Hear Res. 2017;353:8–16.
Kas A, Payoux P, Habert M-O, Malek Z, Cointepas Y, El Fakhri G, et al. Validation of a standardized normalization template for statistical parametric mapping analysis of 123I-FP-CIT images. J Nucl Med. 2007;48:1459–67.
Jokinen P, Helenius H, Rauhala E, Brück A, Eskola O, Rinne JO. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J Nucl Med Off Publ Soc Nucl Med. 2009;50:893–9.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89.
Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8.
Lindvall O, Björklund A. Dopaminergic innervation of the globus pallidus by collaterals from the nigrostriatal pathway. Brain Res. 1979;172:169–73.
Sanchez-Gonzalez MA. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–83.
Gardner EL, Ashby CR. Heterogeneity of the mesotelencephalic dopamine fibers: physiology and pharmacology. Neurosci Biobehav Rev. 2000;24:115–8.
Hosp JA, Luft AR. Dopaminergic meso-cortical projections to M1: role in motor learning and motor cortex plasticity. Front Neurol [Internet]. 2013 [cited 2019 Apr 8];4. Available from: http://journal.frontiersin.org/article/10.3389/fneur.2013.00145/abstract SMASH
Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006;1109:93–107.
Bär K-J, de la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, et al. Functional connectivity and network analysis of midbrain and brainstem nuclei. NeuroImage. 2016;134:53–63.
Premi E, Pilotto A, Garibotto V, Bigni B, Turrone R, Alberici A, et al. Impulse control disorder in PD: a lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat Disord. 2016;30:62–6.
Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207.
Cervenka S, Varrone A, Fransén E, Halldin C, Farde L. PET studies of D2-receptor binding in striatal and extrastriatal brain regions: biochemical support in vivo for separate dopaminergic systems in humans. Synap N Y N. 2010;64:478–85.
Caminiti SP, Presotto L, Baroncini D, Garibotto V, Moresco RM, Gianolli L, et al. Axonal damage and loss of connectivity in nigrostriatal and mesolimbic dopamine pathways in early Parkinson’s disease. NeuroImage Clin. 2017;14:734–40.
Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien L-K, et al. Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci Off J Soc Neurosci. 2010;30:14482–9.
Funding
This work was carried out thanks to the support of the A*MIDEX project (no. ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French Government program, managed by the French National Research Agency (ANR), in the framework of DHU-Imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Verger, A., Horowitz, T., Chawki, M.B. et al. From metabolic connectivity to molecular connectivity: application to dopaminergic pathways. Eur J Nucl Med Mol Imaging 47, 413–424 (2020). https://doi.org/10.1007/s00259-019-04574-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04574-3