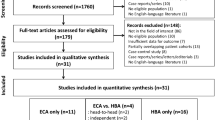
Abstract
To compare the diagnostic value of contrast-enhanced computed tomography (CT) with extracellular contrast agent-enhanced magnetic resonance imaging (ECA-MRI) for the detection of hepatocellular carcinoma (HCC). Pubmed, Embase, Web of Science and Cochrane Library were searched (1/5/2021) for studies comparing contrast-enhanced CT with ECA-MRI in patients suspected of HCC. Studies without head-to-head comparison were excluded. The pooled sensitivity, specificity and summary area under the curve (sAUC) of contrast-enhanced CT and ECA-MRI in detecting HCC was calculated based on bivariate random effects model. Heterogeneity test included threshold effect analysis and meta-regression. Subgroup analyses were conducted according to lesion size (< 20 mm or ≥ 20 mm). Overall, 10 articles containing 1333 patients were deemed suitable for inclusion in this meta-analysis. ECA-MRI displayed increased sensitivity to contrast-enhanced CT in detecting HCC (0.77 vs. 0.63, P < 0.01). The difference in specificity between ECA-MRI and contrast-enhanced CT was not statistically significant (0.93 vs. 0.94, P = 0.25). ECA-MRI yielded higher diagnostic accuracy (sAUCs = 0.88 vs. 0.80, P < 0.01). In the subgroup analysis with a lesion size < 20 mm, ECA-MRI allowed significant gains of accuracy compared to contrast-enhanced CT (0.79 vs. 0.72, P = 0.02). ECA-MRI also outperformed contrast-enhanced CT in patients with lesion size ≥ 20 mm (sAUCs = 0.96 vs. 0.93, P = 0.04). ECA-MRI provided higher sensitivity and accuracy than contrast-enhanced CT in detecting HCC, especially lesions size < 20 mm.
Graphical abstract







Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the author upon reasonable request.
Consent to publication
Not applicable for that section.
References
McGlynn KA, Petrick JL, El‐Serag HB (2021) Epidemiology of hepatocellular carcinoma. Hepatology 73:4–13, doi: https://doi.org/10.1002/hep.31288.
Fu J, Wang H (2018) Precision diagnosis and treatment of liver cancer in China. Cancer Letters 412:283–288, doi: https://doi.org/10.1016/j.canlet.2017.10.008.
Higashi M, Yoshimura T, Usui N et al (2020) A Potential Serum N-glycan Biomarker for Hepatitis C Virus-Related Early-Stage Hepatocellular Carcinoma with Liver Cirrhosis. International Journal of Molecular Sciences 21:8913, doi: https://doi.org/10.3390/ijms21238913.
Liver EAFTSOT (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 69:182–236, doi: https://doi.org/10.1016/j.jhep.2018.03.019.
Lencioni R, Piscaglia F, Bolondi L (2008) Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. Journal of Hepatology 48:848–857, doi: https://doi.org/10.1016/j.jhep.2008.02.005.
Di Martino M, De Filippis G, De Santis A et al (2013) Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. European Radiology 23: 887–96, doi: https://doi.org/10.1007/s00330-012-2691-z.
Lv K, Cao X, Dong Y et al (2021) CT/MRI LI-RADS version 2018 versus CEUS LI-RADS version 2017 in the diagnosis of primary hepatic nodules in patients with high-risk hepatocellular carcinoma. Annals of Translational Medicine 9: 1076, doi: https://doi.org/10.21037/atm-21-1035.
Zhou H, Zhang C, Du L et al (2022) Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Hepatocellular Carcinoma: Diagnostic Performance and Interobserver Agreement. Ultraschall in der medizin 43: 64–71, doi: https://doi.org/10.1055/a-1168-6321.
Manini MA, Sangiovanni A, Fornari F et al (2014) Clinical and economical impact of 2010 AASLD guidelines for the diagnosis of hepatocellular carcinoma. Journal of Hepatology 60:995–1001, doi: https://doi.org/10.1016/j.jhep.2014.01.006.
Hasebroock KM, Serkova NJ (2009) Toxicity of MRI and CT contrast agents. Expert Opin Drug Metab Toxicol, 5: 403–16, doi: https://doi.org/10.1517/17425250902873796.
Vasanawala SS, Alley MT, Hargreaves BA et al (2010) Improved pediatric MR imaging with compressed sensing. Radiology, 256: 607–16, doi: https://doi.org/10.1148/radiol.10091218.
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830, doi: https://doi.org/10.1148/radiol.2018181494.
Roberts LR, Sirlin CB, Zaiem F et al (2018) Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta‐analysis. Hepatology 67:401–421, doi: https://doi.org/10.1002/hep.29487.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6:e1000097, doi: https://doi.org/10.1371/journal.pmed.1000097.
Chang W, Peng F, Meng S-S, Xu J-Y, Yang Y (2020) Diagnostic value of serum soluble triggering expressed receptor on myeloid cells 1 (sTREM-1) in suspected sepsis: a meta-analysis. BMC Immunology 21:1–13, doi: https://doi.org/10.1186/s12865-020-0332-x.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 58:982–990, doi: https://doi.org/10.1016/j.jclinepi.2005.02.022.
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics :837–845, doi: https://doi.org/10.2307/2531595.
Golfieri R, Marini E, Bazzocchi A et al (2009) Small (< or = 3 cm) hepatocellular carcinoma in cirrhosis: the role of double contrast agents in MR imaging vs. multidetector-row CT. Radiologia Medica, 114: 1239–1266, doi: https://doi.org/10.1007/s11547-009-0439-x.
Hassan A, Al-Ajami R, Dashti K, Abdoelmoneum M (2011) Sixty four multi-slice computed tomography and magnetic resonance imaging in evaluation of hepatic focal lesions. Egyptian Journal of Radiology and Nuclear Medicine, 42:101–110, doi: https://doi.org/10.1007/s11547-009-0439-x.
Khalili K, Kim TK, Jang HJ et al (2011) Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. Journal of Hepatology, 54: 723–8, doi: https://doi.org/10.1016/j.jhep.2010.07.025.
Basha MAA, AlAzzazy MZ, Ahmed AF et al (2018) Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. European Radiology, 28: 2592–2603, doi: https://doi.org/10.1007/s00330-017-5232-y.
Min JH, Kim JM, Kim YK et al (2020) Magnetic Resonance Imaging with Extracellular Contrast Detects Hepatocellular Carcinoma with Greater Accuracy Than with Gadoxetic Acid or Computed Tomography. Clinical Gastroenterology and Hepatology, 18: 2091–2100.e7, doi: https://doi.org/10.1016/j.cgh.2019.12.010.
Ronot M, Fouque O, Esvan M et al (2018) Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. Journal of Hepatology, 68: 715–723, doi: https://doi.org/10.1016/j.jhep.2017.12.014.
Leoni S, Piscaglia F, Golfieri R et al (2010) The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. American Journal of Gastroenterology, 105: 599–609, doi: https://doi.org/10.1038/ajg.2009.654.
Sangiovanni A, Manini MA, Iavarone M et al (2010) The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut, 59: 638–44, doi: https://doi.org/10.1136/gut.2009.187286.
Sersté T, Barrau V, Ozenne V et al (2012) Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: role of biopsy. Hepatology, 55: 800–6, doi: https://doi.org/10.1002/hep.24746.
Feng Z, Zhao H, Guan S, Wang W, Rong P (2021) Diagnostic performance of MRI using extracellular contrast agents versus gadoxetic acid for hepatocellular carcinoma: A systematic review and meta‐analysis. Liver International 41:1117–1128, doi: https://doi.org/10.1111/liv.14850.
Li J, Wang J, Lei L, Yuan G, He S (2019) The diagnostic performance of gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced multi-detector computed tomography in detecting hepatocellular carcinoma: a meta-analysis of eight prospective studies. European Radiology 29:6519–6528, doi: https://doi.org/10.1007/s00330-019-06294-6.
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461, doi: https://doi.org/10.1148/radiol.12120826.
Hope TA, Fowler KJ, Sirlin CB et al (2015) Hepatobiliary agents and their role in LI-RADS. Abdominal Imaging 40:613–625, doi: https://doi.org/10.1007/s00261-014-0227-5.
Semaan S, Violi NV, Lewis S et al (2020) Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. European Radiology 30:1020–1030, doi: https://doi.org/10.1007/s00330-019-06458-4.
Tomizawa M, Shinozaki F, Fugo K et al (2017) Diagnostic accuracy of diffusion-weighted whole-body imaging with background body signal suppression/T2-weighted image fusion for the detection of abdominal solid cancer. Experimental and Therapeutic Medicine, 13: 3509–3515, doi: https://doi.org/10.3892/etm.2017.4432.
Kielar AZ, Chernyak V, Bashir MR et al (2018) LI-RADS 2017: An update. Journal of Magnetic Resonance Imaging, 47: 1459–1474, doi: https://doi.org/10.1002/jmri.26027.
Hayashida M, Ito K, Fujita T et al (2008) Small hepatocellular carcinomas in cirrhosis: differences in contrast enhancement effects between helical CT and MR imaging during multiphasic dynamic imaging. Magnetic Resonance Imaging 26:65–71, doi: https://doi.org/10.1016/j.mri.2007.04.007.
Tomemori T, Yamakado K, Nakatsuka A, Sakuma H, Matsumura K, Takeda K (2001) Fast 3D dynamic MR imaging of the liver with MR SmartPrep: comparison with helical CT in detecting hypervascular hepatocellular carcinoma. Clinical Imaging 25:355–361, doi: https://doi.org/10.1016/s0899-7071(01)00332-1.
Bolondi L, Gaiani S, Celli N et al (2005) Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 42:27–34, doi: https://doi.org/10.1002/hep.20728.
Neoplasia ICGfH (2009) Pathologic diagnosis of early hepatocellular carcinoma: a Report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology 49:658–664, doi: https://doi.org/10.1002/hep.22709.
Yamashita Y, Mitsuzaki K, Yi T et al (1996) Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology 200:79–84, doi: https://doi.org/10.1148/radiology.200.1.8657948.
Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR (2016) Differences in liver imaging and reporting data system categorization between MRI and CT. American Journal of Roentgenology 206:307–312, doi: https://doi.org/10.2214/AJR.15.14788.
Lok AS, Perrillo R, Lalama CM et al (2021) Low Incidence of Adverse Outcomes in Adults with Chronic Hepatitis B Virus Infection in the Era of Antiviral Therapy. Hepatology 73:2124–2140, doi: https://doi.org/10.1002/hep.31554.
Bruix J, Ayuso C (2019) Diagnosis of hepatic nodules in patients at risk for hepatocellular carcinoma: LI-RADS probability versus certainty. Gastroenterology 156:860–862, doi: https://doi.org/10.1053/j.gastro.2019.02.008.
Funding
The authors state that this study has received funding by National Natural Science Foundation of China Grant 91959118 (JW), Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-Sen University YHJH201901 (JW), Key Research and Development Program of Guangdong Province 2019B020235002 (JW) and Guangdong Basic and Applied Basic Research Foundation of China Grant 2021A1515010582 (JW).
Author information
Authors and Affiliations
Contributions
Literature search and data extraction were mainly performed by XC and ML. RG helped to conduct study quality assessment. XC and ML performed the study design and data analysis. ML, XC and WL drafted the manuscript. JL, XZ and QC helped to revise the manuscript. JW supervised the study. All authors read and approved the final manuscript. I would like to declare on behalf of my co-authors that the work described was original study that has not been published previously. All the authors listed have approved the manuscript that is enclosed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical approval
Ethics approval was not required because this is a meta-analysis.
Informed consent
The informed consent was waived because this is a meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, X., Li, M., Guo, R. et al. The diagnostic performance of contrast-enhanced CT versus extracellular contrast agent-enhanced MRI in detecting hepatocellular carcinoma: direct comparison and a meta-analysis. Abdom Radiol 47, 2057–2070 (2022). https://doi.org/10.1007/s00261-022-03484-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03484-7