Abstract
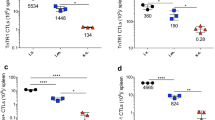
A critical element in improving the potency of cancer vaccines, especially pure protein or peptide antigens, is to develop procedures that can strongly but safely increase their ability to induce immune responses. Here, we describe that encapsulation of a pure protein antigen and interleukin-2 (IL-2) together into liposomes significantly improves immune responses and tumor protection. Groups of C57Bl/6 mice were immunized weekly ×4 with –0.1 mg of ovalbumin (OVA) injected subcutaneously in PBS or encapsulated in liposomes with or without human recombinant IL-2. Control groups included mice immunized to irradiated E.G7-OVA cells (that express ovalbumin), or to PBS. Sera were collected and pooled by immunization group at baseline and at weeks 2 and 4 to measure antibody responses to OVA by ELISA. Splenocytes obtained at week 4 were tested for anti-OVA cellular responses by ELISPOT. Mice were then challenged to a lethal dose of E.G7-OVA cells to measure tumor-protective immunity. IL-2 liposomes caused no detectable toxicity. Antibody, CD8+ T cell, and tumor-protective immune responses were markedly enhanced in mice immunized to OVA + IL-2 in liposomes compared to mice immunized to OVA, either alone or encapsulated into liposomes without IL-2. These results indicate that IL-2 liposomes enhance antibody, cellular, and tumor-protective immune responses to immunization with a soluble protein. This may provide a simple, safe, and effective way to enhance the immunogenicity of vaccines that consist of pure protein antigens.





Similar content being viewed by others
References
Alam A, Imliwati L, Rapthap C, Singh V (2001) Liposome encapsulated tumor-associated antigens elicited humoral and cellular immune responses in mice bearing tumor. Indian J Exp Biol 39:201–208
Akiyama Y, Maruyama K, Nara N, Mochizuki T, Yamamoto A, Yamazaki N, Kawashima I, Nukaya I, Takesako K, Yamaguchi K (2004) Cytotoxic T cell induction against human malignant melanoma cells using HLA-A24-restricted melanoma peptide cocktail. Anticancer Res 24:571–577
Anderson PM, Katsanis E, Spencer SF, Hasz D, Ochoa AC, Bostrom B (1992) Depot characteristics and biodistribution of interleukin-2 liposomes: importance of route of administration. J Immunother 12:19–36
Anderson PM, Hanson DC, Hasz DE, Halet MR, Blazar BR, Ochoa AC (1994) Cytokines in liposomes: preliminary studies with IL-2, IL-6, GM-CSF and interferon-gamma. Cytokine 6:92–101
Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, Dupont B, Chen YT, Hoffman EW, Ritter G, Old LJ, Gnjatic S (2004) Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol 172:3289–3296
Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC (2004) Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest 113:1515–1525
Blattman JN, Greenberg PD (2004) Cancer immunotherapy: a treatment for the masses. Science 305:200–205
Brinkman JA, Fausch SC, Weber JS, Kast WM (2004) Peptide-based vaccines for cancer immunotherapy. Exp Opin Biol Ther 4:181–198
Bystryn J-C, Oratz R, Roses D, Harris M, Henn M, Lew R (1992) Relationship between immune response to melanoma vaccine immunization and clinical outcome in stage II malignant melanoma. Cancer 69:1157–1164
Bystryn JC, Zeleniuch-Jacquotte A, Oratz R, Shapiro RL, Harris MN, Roses DF (2001) Double-blind trial of a polyvalent, shed-antigen, melanoma vaccine. Clin Cancer Res 7:1882–1887
Bystryn JC (2002) Vaccines for melanoma. Dermatol Clin 20:717–725
Davila E, Kennedy R, Celis E (2003) Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res 63:3281–3288
Dermime S, Gilham DE, Shaw DM, Davidson EJ, Meziane el-K, Armstrong A, Hawkins RE, Stern PL (2004) Vaccine and antibody-directed T cell tumour immunotherapy. Biochim Biophys Acta 1704:11–35
Donoline JH, Rosenberg SA (1983) The fate of interleukin-2 after in vivo administration. J Immunol 130:2203–2208
Gershman N, Johnston D, Bystryn J-C (1994) Potentiation of B16 melanoma vaccine immunogenicity by IL-2 liposomes. Vaccine Res 3:83–92
Hanson HL, Kang SS, Norian LA, Matsui K, O’Mara LA, Allen PM (2004) CD4-directed peptide vaccination augments an antitumor response, but efficacy is limited by the number of CD8+ T cell precursors. J Immunol 172:4215–4224
Hsueh EC, Morton DL (2003) Antigen-based immunotherapy of melanoma: canvaxin therapeutic polyvalent cancer vaccine. Semin Cancer Biol 13:401–407
Kast WM, Levitsky H, Marincola FM (2004) Synopsis of the 6th Walker’s cay colloquium on cancer vaccines and immunotherapy. J Transl Med 2(1):20
Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, Kelly DL, Caffrey TC, Hollingsworth MA (2003) Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res 63:5011–5020
Koppenhagen FJ, Kupcu Z, Wallner G, Crommelin DJ, Wagner E, Storm G, Kircheis R (1998) Sustained cytokine delivery for anticancer vaccination: liposomes as alternative for gene-transfected tumor cells. Clin Cancer Res 8:1881–1886
Krup OC, Kroll I, Bose G, Falkenberg FW (1999) Cytokine depot formulations as adjuvants for tumor vaccines. I. Liposome-encapsulated IL-2 as a depot formulation. J Immunother 22:525–538
Lewis JJ (2004) Therapeutic cancer vaccines: using unique antigens. Proc Natl Acad Sci USA 101(Suppl2):14653–14656 Epub
Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G et al (1994) Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol 12:1036–1044
Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C (2004) A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res 64:4987–4994
Lotem M, Shiloni E, Pappo I, Drize O, Hamburger T, Weitzen R, Isacson R, Kaduri L, Merims S, Frankenburg S, Peretz T (2004) Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma. Br J Cancer 90:773–780
Lu J, Higashimoto Y, Appella E, Celis E (2004) Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol 172:4575–4582
Marincola FM, Ferrone S (2003) Immunotherapy of melanoma: the good news, the bad ones and what to do next. Sem Cancer Biol 13:387–389
Miller K, Abeles G, Oratz R, Zeleniuch-Jacquotte A, Cui J, Roses DF, Harris M, Bystryn J-C (1995) Improved survival of melanoma patients with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer 75:495–502
Moore MW, Carbone FR, Bevan MJ (1988) Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777–785
Neville ME, Boni LT, Pflug LE, Popescu MC, Robb RJ (2000) Biopharmaceutics of liposomal interleukin 2, oncolipin. Cytokine 12:1691–1701
Nguyen CL, Salem ML, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ, Gillanders WE (2003) Mechanisms of enhanced antigen-specific T cell response following vaccination with a novel peptide-based cancer vaccine and systemic interleukin-2 (IL-2). Vaccine 21:2318–2328
Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE, Rosenberg SA (2003) Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother 6:349–356
Platsoucas CD, Fincke JE, Pappas J, Jung WJ, Heckel M, Schwarting R, Magira E, Monos D, Freedman RS (2003) Immune responses to human tumors: development of tumor vaccines. Anticancer Res 23:1969–1996
Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn J-C (1998) HLA-independent heterogeneity of CD8+ T cell responses to MAGE-3, Melan A/MART-1, gp100, tyrosinase, MC1R and TRP-2 in vaccine-treated melanoma patients. J Immunol 161:6970–6976
Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn J-C (2000) Identification of HLA-A*03, A*11 and B*07-restricted melanoma-associated peptides that are immunogenic in vivo by vaccine-induced immune response (VIIR) analysis, J Immunol methods 244:59–67
Reynolds SR, Zeleniuch-Jacquotte A, Shapiro RL, Roses DF, Harris MN, Johnston D, Bystryn JC (2003) Vaccine-induced CD8+ T-cell responses to MAGE-3 correlate with clinical outcome in patients with melanoma. Clin Cancer Res 9:657–662
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915
Sato Y, Maeda Y, Shomura H, Sasatomi T, Takahashi M, Une Y, Kondo M, Shinohara T, Hida N, Katagiri K, Sato K, Sato M, Yamada A, Yamana H, Harada M, Itoh K, Todo S (2004) A phase I trial of cytotoxic T-lymphocyte precursor-oriented peptide vaccines for colorectal carcinoma patients. Br J Cancer 90:1334–1342
Slingluff CL Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH (2003) Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol 21:4016–4026
Speiser DE, Rimoldi D, Batard P, Lienard D, Lejeune F, Cerottini JC, Romero P (2003) Disease-driven T cell activation predicts immune responses to vaccination against melanoma. Cancer Immun 3:12
Van Slooten ML, Storm G, Zoephel A, Kupcu Z, Boerman O, Crommelin DJ, Wagner E, Kircheis R (2001) Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm Res 17:42–48
Weinreich DM, Rosenberg SA (2002) Response rates of patients with metastatic melanoma to high dose intravenous interleukin-2 after prior exposure to alpha-interferon or low-dose interleukin-2. J immunother 25:185–187
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grant CA096804 (DJ)
Rights and permissions
About this article
Cite this article
Johnston, D., Reynolds, S.R. & Bystryn, JC. Interleukin-2/liposomes potentiate immune responses to a soluble protein cancer vaccine in mice. Cancer Immunol Immunother 55, 412–419 (2006). https://doi.org/10.1007/s00262-005-0013-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0013-x