Abstract
Introduction
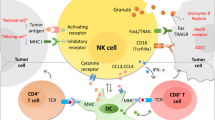
Natural killer cells (NK) are often believed to play a positive role in the antitumor immune response. However, this is not the case for patients with advanced pancreatic cancer. This study was performed to determine the unique subtype of “educated” NK cells and their prognostic value in patients with advanced pancreatic cancer.
Methods
We divided 378 eligible patients into a derivation cohort (September 2010 to December 2014, n = 239) and a validation cohort (January 2015 to April 2016, n = 139). Flow cytometry was performed to analyze NK cells. Enzyme-linked immunosorbent assay was used to detect interleukin-2 (IL-2), interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) production. The Kaplan–Meier method and the Cox proportional hazards model were used.
Results
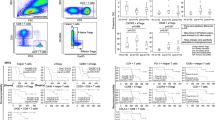
Survival analysis showed that a high density of NK cells accompanied by a high neutrophil-to-lymphocyte ratio was associated with reduced overall survival in both the derivation and validation cohorts. Multivariable analysis also showed that high NK infiltration (HR 1.45, 95% CI 1.17 to 1.79, p = 0.001) was an independent prognostic factor. In these patients, high NK infiltration was associated with reduced levels of IL-2, IFN-γ and TNF-α, although only IFN-γ reached statistical significance, which accounted for this unique phenomenon.
Discussion
Natural killer cells in patients with advanced pancreatic cancer are a unique subtype with anergic features. A high density of NKs predicts poor survival in these patients, possibly because an active inflammatory response and reduced secretion of IL-2, IFN-γ and TNF-α inhibit NK activation.



Similar content being viewed by others
Abbreviations
- CBC:
-
Complete blood counts
- CI:
-
Confidence interval
- FDUSCC:
-
Fudan University Shanghai Cancer Center
- HIS:
-
Hospital Information System
- HR:
-
Hazard ratio
- MMP:
-
Matrix metalloproteinase
- NCAM:
-
Neural cell adhesion molecule
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TRF2:
-
Telomeric repeat binding factor 2
References
Yang C, Liu C, Yu X (2018) Anergic natural killer cells educated by tumor cells are associated with poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Pancreatology 18(4 Supplement):S134 (poster number: P3–104)
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J, Liu L, Liu C, Yu X (2014) Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology 14:295–301
Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I (2011) Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71:5412–5422
Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA (2003) CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101:3052–3057
Costanzo MC, Creegan M, Lal KG, Eller MA (2015) OMIP-027: functional analysis of human natural killer cells. Cytometry A 87:803–805
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil–lymphocyte versus platelet–lymphocyte ratio. Am J Surg 200:197–203
Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP (2011) Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg 35:868–872
Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, Yu X (2015) Blood neutrophil–lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 22:670–676
Kuzman JA, Stenehjem DD, Merriman J, Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM, Agarwal N (2017) Neutrophil–lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma. BMC Urol 17:1
Foley K, Kim V, Jaffee E, Zheng L (2016) Current progress in immunotherapy for pancreatic cancer. Cancer Lett 381:244–251
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M (2013) Increased neutrophil–lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109:416–421
Ben Q, An W, Wang L, Wang W, Yu L, Yuan Y (2015) Validation of the pretreatment neutrophil–lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 44:471–477
Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, Moretta L, Mingari MC, Vitale M (2009) Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 106:20847–20852
Kerdiles Y, Ugolini S, Vivier E (2013) T cell regulation of natural killer cells. J Exp Med 210:1065–1068
Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I (1993) Immune responses in interleukin-2-deficient mice. Science 262:1059–1061
Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM (2012) Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol 188:5054–5062
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12:180–190
Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365:2067–2077
Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311:1924–1927
Lee BK, Kim MJ, Jang HS, Lee HR, Ahn KM, Lee JH, Choung PH, Kim MJ (2008) A high concentration of MMP-2/gelatinase A and MMP-9/gelatinase B reduce NK cell-mediated cytotoxicity against an oral squamous cell carcinoma cell line. In Vivo 22:593–597
Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, Moretta L, Moretta A, Marcenaro E (2017) Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol 139:335–346
Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119:3734–3743
Gonzalez-Gugel E, Saxena M, Bhardwaj N (2016) Modulation of innate immunity in the tumor microenvironment. Cancer Immunol Immunother 65:1261–1268
Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G (2008) Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112:863–875
Le Maux CB, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, Kubin M, Soria JC, Chouaib S, Mami-Chouaib F (2005) NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol 175:5790–5798
Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D (1991) Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349:329–331
Gurlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, Yevsa T, Woller N, Kloos A, Ostroumov D, Armbrecht N, Manns MP, Dombrowski F, Saborowski M, Kleine M, Wirth TC, Oettle H, Ceyhan GO, Esposito I, Calvisi DF, Kubicka S, Kuhnel F (2016) Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer cells. Gastroenterology 151:338–350
Plate JM, Plate AE, Shott S, Bograd S, Harris JE (2005) Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 54:915–925
Funding
This work was supported by National Natural Science Foundation of China Grants (81370065, 81372653), a basic research project of the Science and Technology Commission of Shanghai Municipality (15JC1401200), and the National Science Fund for Distinguished Young Scholars (81625016).
Author information
Authors and Affiliations
Contributions
CY, HC, CL and XY were involved in the study conception and design. CY and HC were involved in the acquisition, analysis, and interpretation of the data. CY drafted the manuscript, and all authors were involved in critical revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval and ethical standards
The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FDUSCC) and met ethical guidelines of the World Medical Association Declaration of Helsinki. Written informed consent was acquired at FDUSCC.
Additional information
The work was published as a poster at the 50th Jubilee Meeting of the European Pancreatic Club (EPC2018), 13–16 June 2018, in Berlin, Germany [1].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, C., Cheng, H., Zhang, Y. et al. Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Cancer Immunol Immunother 67, 1815–1823 (2018). https://doi.org/10.1007/s00262-018-2235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2235-8