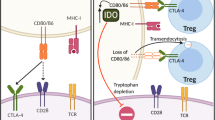
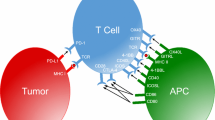
Abstract
Immunotherapy based on checkpoint inhibitors is providing substantial clinical benefit, but only to a minority of cancer patients. The current priority is to understand why the majority of patients fail to respond. Besides T-cell dysfunction, T-cell apoptosis was reported in several recent studies as a relevant mechanism of tumoral immune resistance. Several death receptors (Fas, DR3, DR4, DR5, TNFR1) can trigger apoptosis when activated by their respective ligands. In this review, we discuss the immunomodulatory role of the main death receptors and how these are shaping the tumor microenvironment, with a focus on Fas and its ligand. Fas-mediated apoptosis of T cells has long been known as a mechanism allowing the contraction of T-cell responses to prevent immunopathology, a phenomenon known as activation-induced cell death, which is triggered by induction of Fas ligand (FasL) expression on T cells themselves and qualifies as an immune checkpoint mechanism. Recent evidence indicates that other cells in the tumor microenvironment can express FasL and trigger apoptosis of tumor-infiltrating lymphocytes (TIL), including endothelial cells and myeloid-derived suppressor cells. The resulting disappearance of TIL prevents anti-tumor immunity and may in fact contribute to the absence of TIL that is typical of “cold” tumors that fail to respond to immunotherapy. Interfering with the Fas–FasL pathway in the tumor microenvironment has the potential to increase the efficacy of cancer immunotherapy.


Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cell transfer
- AICD:
-
Activation-induced cell death
- AKT:
-
Protein kinase B
- ALPS:
-
Autoimmune lymphoproliferative Syndrome
- APC:
-
Antigen-presenting cells
- CAF:
-
Cancer-associated fibroblast
- c-FLIP:
-
Cellular FLICE-inhibitory protein
- DD:
-
Death domain
- EMT:
-
Epithelial-to-mesenchymal transition
- FADD:
-
Fas-associated death domain
- FasL:
-
Fas ligand
- GEMM:
-
Genetically engineered mouse model
- HGFR:
-
Hepatocyte growth factor
- IFNγ:
-
Interferon-gamma
- MAGE:
-
Melanoma-associated antigens
- MDM:
-
Monocyte-derived human macrophage
- MMP:
-
Matrix metalloproteinase
- NSCLC:
-
Non-small cell lung cancer
- OPG:
-
Osteoprotegerin
- PD-L2:
-
Programmed death ligand 2
- PMN-MDSC:
-
Polymorphonuclear myeloid-derived suppressor cell
- TAM:
-
Tumor-associated macrophage
- TCRP1A:
-
Anti-P1A T-cell receptor
- TL1A:
-
TNF-like ligand 1A
- TME:
-
Tumor microenvironment
- TRADD:
-
TNF receptor-associated death domain
- Tregs:
-
Regulatory T lymphocytes
- VEGF:
-
Vascular endothelial growth factor
References
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894. https://doi.org/10.1200/JCO.2014.56.2736
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, KEYNOTE-024 Investigators (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867. https://doi.org/10.1056/NEJMoa1602252
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate Investigators (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 1:1. https://doi.org/10.1200/jco.2014.58.3708
Horton BL, Williams JB, Cabanov A, Spranger S, Gajewski TF (2018) Intratumoral CD8(+) T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol Res 6(1):14–24. https://doi.org/10.1158/2326-6066.CIR-17-0249
Zhu J, Powis de Tenbossche CG, Cane S, Colau D, van Baren N, Schmitt-Verhulst AM, Liljestrom P, Uyttenhove C, Van den Eynde B (2017) Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun 8(1):1404
Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S (1991) The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66(2):233–243
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997) The receptor for the cytotoxic ligand TRAIL. Science 276(5309):111–113
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277(5327):818–821
Loetscher H, Schlaeger EJ, Lahm HW, Pan YC, Lesslauer W, Brockhaus M (1990) Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem 265(33):20131–20138
Chinnaiyan AM, O’Rourke K, Yu G-L, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM (1996) Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274:990–992
Pan G, Ni J, Yu G, Wei YF, Dixit VM (1998) TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett 424(1–2):41–45
Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178
Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA (1995) Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189–1192
Nagata S (1997) Apoptosis by death factor. Cell 88(3):355–365
Krammer PH (2000) CD95’s deadly mission in the immune system. Nature 407(6805):789–795. https://doi.org/10.1038/35037728
Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, Kim CH, Kim SJ, Kim JD, Choi IS, Choi IH (1999) Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol 162(4):1889–1895
Tanaka M, Suda T, Takahashi T, Nagata S (1995) Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J 14:1129–1135
O’Reilly L, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, Smyth MJ, Bouillet P, Robb L, Strasser A (2009) Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 461(7264):659–663. https://doi.org/10.1038/nature08402
Nagata S, Suda T (1995) Fas and Fas ligand: lpr and gld mutations. Immunol Today 16(1):39–43
Ramsdell F, Seaman MS, Miller RE, Tough TW, Alderson MR, Lynch DH (1994) gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol 24(4):928–933. https://doi.org/10.1002/eji.1830240422
Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH (1995) Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 181(1):71–77
Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Liénard D, Cerottini J-C, Tschopp J (1996) Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science 274:1363–1366
Restifo NP (2000) Not so Fas: re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med 6:493–495
Seino K, Kayagaki N, Okumura K, Yagita H (1997) Antitumor effect of locally produced CD95 ligand. Nat Med 3(2):165–170
Arai H, Gordon D, Nabel EG, Nabel GJ (1997) Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA 94(25):13862–13867
Ryan AE, Shanahan F, O’Connell J, Houston AM (2005) Addressing the “Fas counterattack” controversy: blocking Fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res 65(21):9817–9823. https://doi.org/10.1158/0008-5472.CAN-05-1462
Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, Dale JK, Fleisher TA, Middelton LA, Sneller MC, Lenardo MJ, Straus SE, Puck JM (1999) Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet 64(4):1002–1014
Boselli D, Losana G, Bernabei P, Bosisio D, Drysdale P, Kiessling R, Gaston JS, Lammas D, Casanova JL, Kumararatne DS, Novelli F (2007) IFN-gamma regulates Fas ligand expression in human CD4+ T lymphocytes and controls their anti-mycobacterial cytotoxic functions. Eur J Immunol 37(8):2196–2204. https://doi.org/10.1002/eji.200636541
Le Gallo M, Poissonnier A, Blanco P, Legembre P (2017) CD95/Fas, non-apoptotic signaling pathways, and kinases. Front Immunol 8:1216. https://doi.org/10.3389/fimmu.2017.01216
Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP (2009) Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA 106(41):17469–17474. https://doi.org/10.1073/pnas.0907448106
Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, Ramaswamy M, Roychoudhuri R, Ji Y, Eil RL, Sukumar M, Crompton JG, Palmer DC, Borman ZA, Clever D, Thomas SK, Patel S, Yu Z, Muranski P, Liu H, Wang E, Marincola FM, Gros A, Gattinoni L, Rosenberg SA, Siegel RM, Restifo NP (2016) Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Investig 126(1):318–334. https://doi.org/10.1172/JCI81217
Kawasaki M, Kuwano K, Nakanishi Y, Hagimoto N, Takayama K, Pei XH, Maeyama T, Yoshimi M, Hara N (2000) Analysis of Fas and Fas ligand expression and function in lung cancer cell lines. Eur J Cancer 36(5):656–663
Ito Y, Monden M, Takeda T, Eguchi H, Umeshita K, Nagano H, Nakamori S, Dono K, Sakon M, Nakamura M, Tsujimoto M, Nakahara M, Nakao K, Yokosaki Y, Matsuura N (2000) The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br J Cancer 82(6):1211–1217. https://doi.org/10.1054/bjoc.1999.1065
Bennett MW, O’Connell J, O’Sullivan GC, Brady C, Roche D, Collins JK, Shanahan F (1998) The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol 160(11):5669–5675
O’Connell J, O’Sullivan GC, Collins JK, Shanahan F (1996) The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 184:1075–1082
Walker PR, Saas P, Dietrich PY (1998) Tumor expression of Fas ligand (CD95L) and the consequences. Curr Opin Immunol 10(5):564–572
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(Pt 19):3365–3374
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172
Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP (2005) Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 35(2):169–173. https://doi.org/10.1016/j.bcmd.2005.07.001
Lin HC, Lai PY, Lin YP, Huang JY, Yang BC (2012) Fas ligand enhances malignant behavior of tumor cells through interaction with Met, hepatocyte growth factor receptor, in lipid rafts. J Biol Chem 287(24):20664–20673. https://doi.org/10.1074/jbc.M111.326058
Merz C, Strecker A, Sykora J, Hill O, Fricke H, Angel P, Gieffers C, Peterziel H (2015) Neutralization of the CD95 ligand by APG101 inhibits invasion of glioma cells in vitro. Anticancer Drugs 26(7):716–727. https://doi.org/10.1097/CAD.0000000000000237
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, Schreglmann N, Letellier E, Zuliani C, Klussmann S, Teodorczyk M, Grone HJ, Ganten TM, Sultmann H, Tuttenberg J, von Deimling A, Regnier-Vigouroux A, Herold-Mende C, Martin-Villalba A (2008) Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 13(3):235–248. https://doi.org/10.1016/j.ccr.2008.02.003
Steller EJ, Borel Rinkes IH, Kranenburg O (2011) How CD95 stimulates invasion. Cell Cycle 10(22):3857–3862 https://doi.org/10.4161/cc.10.22.18290
Wisniewski P, Ellert-Miklaszewska A, Kwiatkowska A, Kaminska B (2010) Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP-2 pathway. Cell Signal 22(2):212–220. https://doi.org/10.1016/j.cellsig.2009.09.016
Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61(6):2744–2750
Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, Wiestler B, Schliesser MG, von Deimling A, Pichler J, Vetlova E, Harting I, Debus J, Hartmann C, Kunz C, Platten M, Bendszus M, Combs SE (2014) A phase II, randomized, study of weekly APG101 + reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res 20(24):6304–6313. https://doi.org/10.1158/1078-0432.CCR-14-0951-T
Teodorczyk M, Kleber S, Wollny D, Sefrin JP, Aykut B, Mateos A, Herhaus P, Sancho-Martinez I, Hill O, Gieffers C, Sykora J, Weichert W, Eisen C, Trumpp A, Sprick MR, Bergmann F, Welsch T, Martin-Villalba A (2015) CD95 promotes metastatic spread via Sck in pancreatic ductal adenocarcinoma. Cell Death Differ 22(7):1192–1202. https://doi.org/10.1038/cdd.2014.217
Dudley AC (2012) Tumor endothelial cells. Cold Spring Harb Perspect Med 2(3):a006536. https://doi.org/10.1101/cshperspect.a006536
Yu JS, Lee PK, Ehtesham M, Samoto K, Black KL, Wheeler CJ (2003) Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol 64(1–2):55–61
Bajou K, Peng H, Laug WE, Maillard C, Noel A, Foidart JM, Martial JA, DeClerck YA (2008) Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell 14(4):324–334. https://doi.org/10.1016/j.ccr.2008.08.012
Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G (2014) Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 20(6):607–615. https://doi.org/10.1038/nm.3541
Tischner D, Woess C, Ottina E, Villunger A (2010) Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Dis 1:e48. https://doi.org/10.1038/cddis.2010.27
Tischner D, Gaggl I, Peschel I, Kaufmann M, Tuzlak S, Drach M, Thuille N, Villunger A, Jan Wiegers G (2012) Defective cell death signalling along the Bcl-2 regulated apoptosis pathway compromises Treg cell development and limits their functionality in mice. J Autoimmun 38(1):59–69. https://doi.org/10.1016/j.jaut.2011.12.008
Veglia F, Perego M, Gabrilovich D (2018) Myeloid-derived suppressor cells coming of age. Nat Immunol 19(2):108–119. https://doi.org/10.1038/s41590-017-0022-x
Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P (2000) Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96(12):3838–3846
Kusmartsev SA, Li Y, Chen SH (2000) Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol 165(2):779–785
Condamine T, Ramachandran I, Youn JI, Gabrilovich DI (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97–110. https://doi.org/10.1146/annurev-med-051013-052304
Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S (2011) Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood 117(20):5381–5390. https://doi.org/10.1182/blood-2010-11-321752
Weiss JM, Subleski JJ, Back T, Chen X, Watkins SK, Yagita H, Sayers TJ, Murphy WJ, Wiltrout RH (2014) Regulatory T cells and myeloid-derived suppressor cells in the tumor microenvironment undergo Fas-dependent cell death during IL-2/alphaCD40 therapy. J Immunol 192(12):5821–5829. https://doi.org/10.4049/jimmunol.1400404
Peyvandi S, Buart S, Samah B, Vetizou M, Zhang Y, Durrieu L, Polrot M, Chouaib S, Benihoud K, Louache F, Karray S (2015) Fas ligand deficiency impairs tumor immunity by promoting an accumulation of monocytic myeloid-derived suppressor cells. Cancer Res 75(20):4292–4301. https://doi.org/10.1158/0008-5472.CAN-14-1848
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW (2013) Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med 19(4):465–472. https://doi.org/10.1038/nm.3105
Huijbers IJ, Krimpenfort P, Chomez P, van der Valk MA, Song JY, Inderberg-Suso EM, Schmitt-Verhulst AM, Berns A, Van den Eynde BJ (2006) An inducible mouse model of melanoma expressing a defined tumor antigen. Cancer Res 66(6):3278–3286
Wehbe M, Soudja SM, Mas A, Chasson L, Guinamard R, de Tenbossche CP, Verdeil G, Van den Eynde B, Schmitt-Verhulst AM (2012) Epithelial–mesenchymal-transition-like and TGFbeta pathways associated with autochthonous inflammatory melanoma development in mice. PLoS One 7(11):e49419. https://doi.org/10.1371/journal.pone.0049419
Soudja SM, Wehbe M, Mas A, Chasson L, de Tenbossche CP, Huijbers I, Van den Eynde B, Schmitt-Verhulst AM (2010) Tumor-initiated inflammation overrides protective adaptive immunity in an induced melanoma model in mice. Cancer Res 70:3515–3525. https://doi.org/10.1158/0008-5472.can-09-4354
Seino K, Iwabuchi K, Kayagaki N, Miyata R, Nagaoka I, Matsuzawa A, Fukao K, Yagita H, Okumura K (1998) Chemotactic activity of soluble Fas ligand against phagocytes. J Immunol 161(9):4484–4488
Ottonello L, Tortolina G, Amelotti M, Dallegri F (1999) Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol 162(6):3601–3606
Hohlbaum AM, Moe S, Marshak-Rothstein A (2000) Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med 191(7):1209–1220
Shudo K, Kinoshita K, Imamura R, Fan H, Hasumoto K, Tanaka M, Nagata S, Suda T (2001) The membrane-bound but not the soluble form of human Fas ligand is responsible for its inflammatory activity. Eur J Immunol 31(8):2504–2511. https://doi.org/10.1002/1521-4141(200108)31:8%3c2504:AID-IMMU2504%3e3.0.CO;2-C
Chen YL, Chen SH, Wang JY, Yang BC (2003) Fas ligand on tumor cells mediates inactivation of neutrophils. J Immunol 171(3):1183–1191
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10(15):4895–4900. https://doi.org/10.1158/1078-0432.CCR-03-0760
Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P (2001) The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 97(2):339–345
Moses K, Brandau S (2016) Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol 28(2):187–196. https://doi.org/10.1016/j.smim.2016.03.018
Fridlender ZG, Albelda SM (2012) Tumor-associated neutrophils: friend or foe? Carcinogenesis 33(5):949–955. https://doi.org/10.1093/carcin/bgs123
Zhou J, Nefedova Y, Lei A, Gabrilovich D (2018) Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol 35:19–28. https://doi.org/10.1016/j.smim.2017.12.004
Dockrell DH, Badley AD, Villacian JS, Heppelmann CJ, Algeciras A, Ziesmer S, Yagita H, Lynch DH, Roche PC, Leibson PJ, Paya CV (1998) The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J Clin Investig 101(11):2394–2405. https://doi.org/10.1172/JCI1171
Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV (2007) Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26(5):629–641. https://doi.org/10.1016/j.immuni.2007.03.016
Ashany D, Savir A, Bhardwaj N, Elkon KB (1999) Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol 163(10):5303–5311
Willems F, Amraoui Z, Vanderheyde N, Verhasselt V, Aksoy E, Scaffidi C, Peter ME, Krammer PH, Goldman M (2000) Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood 95(11):3478–3482
Rescigno M, Piguet V, Valzasina B, Lens S, Zubler R, French L, Kindler V, Tschopp J, Ricciardi-Castagnoli P (2000) Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J Exp Med 192(11):1661–1668
Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD (2018) Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun 9(1):948. https://doi.org/10.1038/s41467-018-03347-0
Strauss L, Bergmann C, Whiteside TL (2009) Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol 182(3):1469–1480
Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, Krammer P, Linderkamp O, Suri-Payer E (2006) Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood 108(10):3371–3378. https://doi.org/10.1182/blood-2006-02-005660
Plaza-Sirvent C, Schuster M, Neumann Y, Heise U, Pils MC, Schulze-Osthoff K, Schmitz I (2017) c-FLIP expression in Foxp3-expressing cells is essential for survival of regulatory T cells and prevention of autoimmunity. Cell Rep 18(1):12–22. https://doi.org/10.1016/j.celrep.2016.12.022
Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G (2011) Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 133(2):190–196. https://doi.org/10.1111/j.1365-2567.2011.03426.x
Kameoka M, Suzuki S, Kimura T, Fujinaga K, Auwanit W, Luftig RB, Ikuta K (1997) Exposure of resting peripheral blood T cells to HIV-1 particles generates CD25+ killer cells in a small subset, leading to induction of apoptosis in bystander cells. Int Immunol 9(10):1453–1462
Hahn S, Gehri R, Erb P (1995) Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev 146:57–79
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
LeBlanc HN, Ashkenazi A (2003) Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ 10(1):66–75. https://doi.org/10.1038/sj.cdd.4401187
Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS (1999) Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res 59(12):2770–2775
Oikonomou E, Kothonidis K, Taoufik E, Probert E, Zografos G, Nasioulas G, Andera L, Pintzas A (2007) Newly established tumourigenic primary human colon cancer cell lines are sensitive to TRAIL-induced apoptosis in vitro and in vivo. Br J Cancer 97(1):73–84. https://doi.org/10.1038/sj.bjc.6603835
Walczak H (2013) Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol 5(5):a008698. https://doi.org/10.1101/cshperspect.a008698
Liguori M, Buracchi C, Pasqualini F, Bergomas F, Pesce S, Sironi M, Grizzi F, Mantovani A, Belgiovine C, Allavena P (2016) Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget 7(27):41662–41676. https://doi.org/10.18632/oncotarget.9340
Wendling U, Walczak H, Dorr J, Jaboci C, Weller M, Krammer PH, Zipp F (2000) Expression of TRAIL receptors in human autoreactive and foreign antigen-specific T cells. Cell Death Differ 7(7):637–644. https://doi.org/10.1038/sj.cdd.4400692
Jeremias I, Herr I, Boehler T, Debatin KM (1998) TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol 28(1):143–152. https://doi.org/10.1002/(SICI)1521-4141(199801)28:01%3c143:AID-IMMU143%3e3.0.CO;2-3
Lunemann JD, Waiczies S, Ehrlich S, Wendling U, Seeger B, Kamradt T, Zipp F (2002) Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto)antigen-specific T cells. J Immunol 168(10):4881–4888
Chyuan IT, Tsai HF, Wu CS, Sung CC, Hsu PN (2018) TRAIL-mediated suppression of T cell receptor signaling inhibits T cell activation and inflammation in experimental autoimmune encephalomyelitis. Front Immunol 9:15. https://doi.org/10.3389/fimmu.2018.00015
Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI (2017) Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res 23(12):2942–2950. https://doi.org/10.1158/1078-0432.CCR-16-1784
Diao Z, Shi J, Zhu J, Yuan H, Ru Q, Liu S, Liu Y, Zheng D (2013) TRAIL suppresses tumor growth in mice by inducing tumor-infiltrating CD4(+)CD25 (+) Treg apoptosis. Cancer Immunol Immunother 62(4):653–663. https://doi.org/10.1007/s00262-012-1370-x
Vandenabeele P, Declercq W, Beyaert R, Fiers W (1995) Two tumour necrosis factor receptors: structure and function. Trends Cell Biol 5(10):392–399
Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3(9):745–756. https://doi.org/10.1038/nri1184
Naude PJ, den Boer JA, Luiten PG, Eisel UL (2011) Tumor necrosis factor receptor cross-talk. FEBS J 278(6):888–898. https://doi.org/10.1111/j.1742-4658.2011.08017.x
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N (2008) Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Investig 118(2):560–570. https://doi.org/10.1172/JCI32453
Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, Kato N (2003) Silk protein, sericin, suppresses DMBA-TPA-induced mouse skin tumorigenesis by reducing oxidative stress, inflammatory responses and endogenous tumor promoter TNF-alpha. Oncol Rep 10(3):537–543
Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR (2003) An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther 2(5):445–451
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z (2012) TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Investig 122(11):4094–4104. https://doi.org/10.1172/JCI64115
Chen X, Oppenheim JJ (2011) Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett 585(23):3611–3618. https://doi.org/10.1016/j.febslet.2011.04.025
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR (1998) DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1(4):543–551
Pobezinskaya YL, Choksi S, Morgan MJ, Cao X, Liu ZG (2011) The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling. J Immunol 186(9):5212–5216. https://doi.org/10.4049/jimmunol.1002374
Zeng L, Li T, Xu DC, Liu J, Mao G, Cui MZ, Fu X, Xu X (2012) Death receptor 6 induces apoptosis not through type I or type II pathways, but via a unique mitochondria-dependent pathway by interacting with Bax protein. J Biol Chem 287(34):29125–29133. https://doi.org/10.1074/jbc.M112.362038
Vanamee ES, Faustman DL (2017) TNFR2: a novel target for cancer immunotherapy. Trends Mol Med 23(11):1037–1046. https://doi.org/10.1016/j.molmed.2017.09.007
Kunkele A, Johnson AJ, Rolczynski LS, Chang CA, Hoglund V, Kelly-Spratt KS, Jensen MC (2015) Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell Fas–FasL-dependent AICD. Cancer Immunol Res 3(4):368–379. https://doi.org/10.1158/2326-6066.CIR-14-0200
Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, Du L, Jiang J, Wu C, He X, Roberts AI, Li F, Rabson AB, Wang Y, Shi Y (2015) Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene 34(49):5960–5970. https://doi.org/10.1038/onc.2015.46
Gastman BR, Johnson DE, Whiteside TL, Rabinowich H (2000) Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood 95(6):2015–2023
Acknowledgements
We are grateful to Ms. Auriane Sibille for her precious help in the preparation of this manuscript.
Funding
Pierre-Florent Petit is supported by a fellowship from the Fonds National de la Recherche Scientifique (FNRS-Aspirant grant No. 1.A.818.18).
Author information
Authors and Affiliations
Contributions
Jingjing Zhu and Benoit J. Van den Eynde conceived the manuscript. Pierre-Florent Petit designed the figures. All authors contributed to writing and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, J., Petit, PF. & Van den Eynde, B.J. Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother 68, 835–847 (2019). https://doi.org/10.1007/s00262-018-2269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2269-y