Abstract
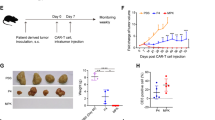
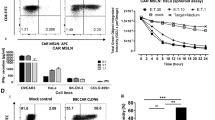
The interaction between programmed cell death protein 1 (PD-1) on activated T cells and its ligands on a target tumour may limit the capacity of chimeric antigen receptor (CAR) T cells to eradicate solid tumours. PD-1 blockade could potentially enhance CAR T cell function. Here, we show that mesothelin is overexpressed in human triple-negative breast cancer cells and can be targeted by CAR T cells. To overcome the suppressive effect of PD-1 on CAR T cells, we utilized CRISPR/Cas9 ribonucleoprotein-mediated editing to disrupt the programmed cell death-1 (PD-1) gene locus in human primary T cells, resulting in a significantly reduced PD-1hi population. This reduction had little effect on CAR T cell proliferation but strongly augmented CAR T cell cytokine production and cytotoxicity towards PD-L1-expressing cancer cells in vitro. CAR T cells with PD-1 disruption show enhanced tumour control and relapse prevention in vivo when compared with CAR T cells with or without αPD-1 antibody blockade. Our study demonstrates a potential advantage of integrated immune checkpoint blockade with CAR T cells in controlling solid tumours and provides an alternative CAR T cell strategy for adoptive transfer therapy.






Similar content being viewed by others
Abbreviations
- 4-1BB TNF:
-
Receptor superfamily member 9
- CRISPR/Cas9:
-
sgRNA-guided clustered regularly interspaced short palindrome repeats-associated nuclease Cas9
- Meso:
-
Mesothelin
- RFP:
-
Red fluorescence protein
- RNP:
-
Ribonucleoprotein
- T7E1:
-
T7 endonuclease I
- Tim-3:
-
T cell immunoglobulin and mucin-domain-containing-3
- TNBC:
-
Triple-negative breast cancer
References
Gross G, Waks T, Eshhar Z (1989) Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA 86(24):10024–10028
Johnson LA, June CH (2017) Driving gene-engineered T cell immunotherapy of cancer. Cell Res 27(1):38–58
Gross G, Eshhar Z (2016) Therapeutic potential of T cell chimeric antigen receptors (CARs) in cancer treatment: counteracting off-tumor toxicities for safe CAR T cell therapy. Annu Rev Pharmacol Toxicol 56:59–83
Bera TK, Pastan I (2000) Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 20(8):2902–2906
Morello A, Sadelain M, Adusumilli PS (2016) Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 6(2):133–146
Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH (2014) Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2(2):112–120
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121(7):2750–2767
Parinyanitikul N, Blumenschein GR, Wu Y, Lei X, Chavez-Macgregor M, Smart M, Gonzalez-Angulo AM (2013) Mesothelin expression and survival outcomes in triple receptor negative breast cancer. Clin Breast Cancer 13(5):378–384
Tchou J, Wang LC, Selven B, Zhang H, Conejo-Garcia J, Borghaei H, Kalos M, Vondeheide RH, Albelda SM, June CH, Zhang PJ (2012) Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat 133(2):799–804
Lamberts LE, de Groot DJ, Bense RD, de Vries EG, Fehrmann RS (2015) Functional genomic mRNA profiling of a large cancer data base demonstrates mesothelin overexpression in a broad range of tumor types. Oncotarget 6(29):28164–28172
Tozbikian G, Brogi E, Kadota K, Catalano J, Akram M, Patil S, Ho AY, Reis-Filho JS, Weigelt B, Norton L, Adusumilli PS, Wen HY (2014) Mesothelin expression in triple negative breast carcinomas correlates significantly with basal-like phenotype, distant metastases and decreased survival. PloS One 9(12):e114900
Motz GT, Coukos G (2013) Deciphering and reversing tumor immune suppression. Immunity 39(1):61–73
Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL (2013) Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA 110(27):E2480–E2489
Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, June CH, Riley JL, Wherry EJ, Albelda SM (2014) Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric Antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20(16):4262–4273
Callahan MK, Postow MA, Wolchok JD (2016) Targeting T cell co-receptors for cancer therapy. Immunity 44(5):1069–1078
Borch TH, Donia M, Andersen MH, Svane IM (2015) Reorienting the immune system in the treatment of cancer by using anti-PD-1 and anti-PD-L1 antibodies. Drug Discov Today 20(9):1127–1134
Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ (2017) In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aal3604
Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y, Moon EK (2016) A Chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 76(6):1578–1590
Chen N, Morello A, Tano Z, Adusumilli PS (2017) CAR T-cell intrinsic PD-1 checkpoint blockade: a two-in-one approach for solid tumor immunotherapy. Oncoimmunology 6(2):e1273302
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Investig 126(8):3130–3144
Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, Marson A (2015) Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA 112(33):10437–10442
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ (2017) PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129(8):1039–1041
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y (2017) Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res 23(9):2255–2266
Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A (2017) CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep 7(1):737
Su S, Zou Z, Chen F, Ding N, Du J, Shao J, Li L, Fu Y, Hu B, Yang Y, Sha H, Meng F, Wei J, Huang X, Liu B (2017) CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology 6(1):e1249558
Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A, Boesteanu AC, Cogdill AP, Chen T, Fraietta JA, Kloss CC, Posey AD Jr, Engels B, Singh R, Ezell T, Idamakanti N, Ramones MH, Li N, Zhou L, Plesa G, Seykora JT, Okada H, June CH, Brogdon JL, Maus MV (2015) Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 7(275):275ra222
Zhang Q, Wei F, Wang HY, Liu X, Roy D, Xiong QB, Jiang S, Medvec A, Danet-Desnoyers G, Watt C, Tomczak E, Kalos M, Riley JL, Wasik MA (2013) The potent oncogene NPM-ALK mediates malignant transformation of normal human CD4(+) T lymphocytes. Am J Pathol 183(6):1971–1980
Chowdhury PS, Viner JL, Beers R, Pastan I (1998) Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA 95(2):669–674
Chang K, Pastan I (1996) Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 93(1):136–140
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH (2009) Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA 106(9):3360–3365
Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M (2015) Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer cell 28(4):415–428
Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J, Keith B, Snyder NW, Blair IA, Milone MC, June CH (2016) Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44(2):380–390
Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, Zhou L, Loew A, Young RM, June CH, Zhao Y (2015) Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res 75(17):3596–3607
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161(2):205–214
Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y (2017) A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8(10):17002–17011
Inaguma S, Wang Z, Lasota J, Onda M, Czapiewski P, Langfort R, Rys J, Szpor J, Waloszczyk P, Okon K, Biernat W, Ikeda H, Schrump DS, Hassan R, Pastan I, Miettinen M (2017) Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget 8(16):26744–26754
Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, Dengel K, Kerr ND, Bagg A, Levine BL, June CH, Stadtmauer EA (2015) Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 373(11):1040–1047
Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, Youngblood B (2017) De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170(1):142–157 e119
Acknowledgements
We are grateful to the blood donors for their contribution; Zhouluo Ou for providing the breast cancer cell lines used in this study; Ilya Vinnikov for proofreading the manuscript; and Wei Zhang and Huan Wang for their helpful suggestions.
Funding
This work was supported by the National Natural Science Foundation of China (81402542) and the scholarship of Pujiang Talents in Shanghai to Fang Wei (14PJ1405600).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design of this work. WH, ZZ and YJ performed the experiments, analysed the data and wrote the manuscript. KS contributed to blood donor communication and sample collection. GL contributed to the molecular biology experiments. QC and XM proofread the manuscript and gave intellectual suggestions. FW contributed to the conception and design, data analysis and interpretation, and manuscript writing and provided financial support. All authors viewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
For the use of blood samples from healthy donors, written informed consent was obtained in accordance with the of the Shanghai Jiao Tong Human Sample Committee on March 1, 2014. The mouse study was carried out in accordance with the recommendations of East China Normal University Animal Care guidelines from the East China Normal University Animal Care Committee. All experimental protocols were approved on August 1, 2016, by the East China Normal University Animal Care Committee.
Animal source
Six-week-old female NOD-Prkdcscid IL2rgnull (NSG) mice were purchased from Vitalstar, China, and housed in ventilated cages in our pathogen-free facility.
Cell line authentication
The human cell lines MCF7, MDA-MB-231, BT-549, Hs 578T and HeLa were kindly provided by Zouluo Ou (Fudan University, Shanghai, China). The cell lines were authenticated by the Chinese Academy of Sciences Committee Type Culture Collection Cell Bank.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, W., Zi, Z., Jin, Y. et al. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother 68, 365–377 (2019). https://doi.org/10.1007/s00262-018-2281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2281-2