Abstract
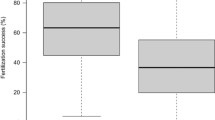
Genes of the major histocompatibility complex (MHC) are remarkably polymorphic. Several selection mechanisms have been invoked to account for this diversity, including disassortative mating preferences. In addition, eggs may discriminate between sperm based on MHC. To investigate the effects of MHC-genotype on fertilization success, we obtained mature gametes from ripe Arctic charr (Salvelinus alpinus) males and females captured on spawning grounds. The eggs of each female were divided into two batches, and by letting each of 2 males fertilize 1 of the batches, we obtained a total of 36 half-sibling batch-pairs. The semen was diluted to ensure that the two males in each half-sibling batch-pair contributed with the same number of sperm cells. We found that MHC-heterozygous males had significantly higher fertilization success than MHC-homozygous males and neither initial spermatocrit, sperm motility nor swimming velocity co-varied with difference in fertilization success. There was no effect of female genotype or female-male MHC-similarity on fertilization success. However, one MHC-allele was associated with increased fertilization success. It seems plausible that the difference in fertilization success between homo- and heterozygous males may be due to MHC-dependent sperm selection by the ovum.



Similar content being viewed by others
References
Amanze D, Iyengar A (1990) The micropyle: a sperm guidance system in teleost fertilization. Development 109:495–500
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Apanius V, Penn D, Slev PR, Ruff LR, Potts W (1997) The nature of selection on the major histocompatibility complex. Crit Rev Immunol 17:179–224
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic, London
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3:262–273
Carré D, Sardet C (1984) Fertilization and early development in Beroe ovata. Dev Biol 105:188–195
Cohen J (1991) The case for and against sperm selection. In: Bacetti B (ed) Comparative spermatology 20 years after. Raven, New York, pp 759–764
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Egid K, Brown JL (1989) The major histocompatibility complex and female mating preferences in mice. Anim Behav 38:548–550
Fisher S, Lerman LS (1983) DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci 80:1579–1583
Hamilton WD, Poulin R (1997) The Hamilton and Zuk hypothesis revisited: a meta-analytical approach. Behaviour 134:299–320
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Janeway CA, Travers P (1997) Immunobiology: the immune system in health and disease. Garland, New York
Johnson L (1980) The Arctic charr, Salvelinus alpinus. In: Balon EK (ed) Charrs, salmonid fishes of the genus Salvelinus. Junk, The Hague, pp 15–98
Jonsson B, Jonsson N (2001) Polymorphism and speciation in the Arctic charr. J Fish Biol 58:605–638
Jordan WC, Bruford MW (1998) New perspectives on mate choice and the MHC. Heredity 81:239–245
Kamler E (1992) Early life history of fish: an energetics approach. Chapman & Hall, London
Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A (1991) Simplified mammalian DNA isolation procedure. Nucl Acid Res 19:4293
Langefors Å, Lohm J, von Schantz T, Grahn M (2000) Screening of Mhc variation in Atlantic salmon (Salmo salar): a comparison of restriction fragment length polymorphism (RFLP), denaturing gradient gel electrophoresis (DGGE) and sequencing. Mol Ecol 9:215–219
Miller KM, Withler RE (1996) Sequence analysis of a polymorphic Mhc class II gene in Pacific salmon. Immunogenetics 43:337–351
Miller KM, Withler RE (1997) Mhc diversity in Pacific salmon: population structure and trans-species allelism. Hereditas 127:83–95
Myers RM, Maniatis T, Lerman LS (1987) Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol 155:501–527
Ober C, Weitkamp LR, Cox N, Dytch H, Kostyu D, Elias S (1994) HLA and mate choice in humans. Am J Hum Genet 61:497–504
Olsén KH, Grahn M, Lohm J, Langefors Å (1998) MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.). Anim Behav 56:319–327
Penn DJ (2002) The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology 108:1–21
Potts WK, Manning CJ, Wakeland EK (1991) Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352:619–621
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice VA, Andrews FN, Warwick EJ, Legates JE (1967) Breeding and improvement of farm animals. McGraw-Hill, New York
Rülicke T, Chapuisat M, Homberger FR, Macas E, Wedekind C (1998) MHC-genotype of progeny influenced by parental infection. Proc R Soc Lond B 265:711–716
Schantz T von, Wittzell H, Göransson G, Grahn M, Persson K (1996) MHC genotype and male ornamentation: genetic evidence for the Hamilton-Zuk model. Proc R Soc Lond B 263:265–271
Siva-Jothy MT, Skarstein F (1998) Towards a functional understanding of “good genes”. Ecol Lett 1:178–185
Stockley P (1997) No evidence of sperm selection by female in common shrews. Proc R Soc Lond B 264:1497–1500
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Urbach D, Folstad I, Rudolfsen G (2004) Sperm motility in ovarian fluid: Cryptic female choice in arctic charr? Behav Ecol Sociobiol (in press)
Wedekind C, Folstad I (1994) Adaptive and non-adaptive immunosuppression by sex hormones. Am Nat 143:936–938
Wedekind C, Furi S (1997) Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc R Soc Lond B 264:1471–1479
Wedekind C, Seebeck T, Bettens F, Paepke AJ (1995) MHC-dependent mate preferences in humans. Proc R Soc Lond B 260:245–249
Wildt DE, Bush M, Goodrowe KL, Packer C, Pusey AE, Brown JL, Joslin P, O’Brien SJ (1987) Reproductive and genetic consequences of founding isolated populations. Nature 329:328–331
Wilson N, Tubman SC, Eady PE, Robertson GW (1997) Female genotype affects male success in sperm competition. Proc R Soc Lond B 264:1491–1495
Wyrobek AJ (1979) Changes in mammalian sperm morphology after x-ray and chemical exposures. Genetics 92:105–119
Yamazaki K, Boyse EA, Miké V, Mathieson BJ, Abbot BJ, Boyse J, Zayas ZA (1976) Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med 144:1324–1335
Ziegler A, Dohr G, Uchanska-Ziegler B (2002) Possible roles for products of polymorphic MHC and linked olfactory receptor genes during selection processes in reproduction. Am J Reprod Immunol 48:34–42
Acknowledgements
We are grateful for the constructive comments of Claus Wedekind, Jakob Lohm and several anonymous referees, and the patience of editor Mark Abrahams.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Abrahams
Rights and permissions
About this article
Cite this article
Skarstein, F., Folstad, I., Liljedal, S. et al. MHC and fertilization success in the Arctic charr (Salvelinus alpinus). Behav Ecol Sociobiol 57, 374–380 (2005). https://doi.org/10.1007/s00265-004-0860-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0860-z