Abstract
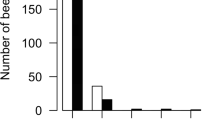
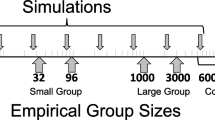
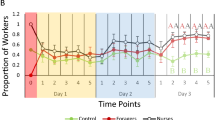
Behavioral specialization and cooperation are fundamental in the organization and success of social groups. Honey bee workers display hygienic behavior, defined as the detection, uncapping, and removal of unhealthy brood. We present detailed analyses of behavioral specialization and task partitioning among hygienic worker bees, focusing on uncapping of brood cells and removal of the cells’ content in freeze-killed brood assays. We demonstrate specialization of hygienic workers on either uncapping or removal and task partitioning among multiple individuals. Speed of hygiene decreases with the number of behavioral instances, suggesting a time cost for cooperation of multiple individuals. Additional analyses of an individual agent-based simulation of hygienic removal of Varroa-mite-infested brood demonstrate that erroneous removal of healthy brood can be reduced by task partitioning due to collective decision-making. Combined, our results indicate a speed-accuracy trade-off in the collective performance of hygienic behavior: Hygienic behavior may take longer when many individuals contribute and specialize on different tasks, but this organization of work also can prevent costly mistakes. This trade-off may explain the observed combination of elite workers and numerous other workers that contribute only little.
Significance statement
Honey bees and other social animals form successful groups that are characterized by individual specialization on different tasks. Hygienic behavior of honey bees removes unhealthy brood from the nest and is important for the health of honey bee colonies. Here, we observed that individual worker bees specialize on either of the two main tasks involved in hygienic behavior: uncapping cells or removing brood. We also found that a few individuals perform the majority of the work and that the speed of hygienic behavior is higher when one individual works continuously instead of multiple individuals. Additionally, we show in computer simulations that the number of erroneous brood removals is decreased when separate workers contribute to the different aspects of hygienic behavior. Thus, our study indicates that a speed-accuracy trade-off might drive the evolution of task partitioning in hygienic behavior.






Similar content being viewed by others
Data availability
Behavioral data are available as online supplement. Biological materials were not preserved. The simulation code is also provided as online supplement.
References
Al Toufailia H, Evison SE, Hughes WO, Ratnieks FL (2018) Both hygienic and non-hygienic honeybee, Apis mellifera, colonies remove dead and diseased larvae from open brood cells. Phil Trans R Soc B 373:20170201
Anderson C, Ratnieks FL (1999) Task partitioning in insect societies. I. Effect of colony size on queueing delay and colony ergonomic efficiency. Am Nat 154:521–535
Anderson C, Ratnieks FLW (2000) Task partitioning in insect societies: novel situations. Ins Soc 47:198–199
Arathi HS, Spivak M (2001) Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee, Apis mellifera L. Anim Behav 62:57–66
Arathi H, Burns I, Spivak M (2000) Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioural repertoire of hygienic bees. Ethology 106:365–379
Arathi HS, Ho G, Spivak M (2006) Inefficient task partitioning among nonhygienic honeybees, Apis mellifera L., and implications for disease transmission. Anim Behav 72:431–438
Baudier KM, Ostwald MM, Grüter C, Segers FHID, Roubik DW, Pavlic TP, Pratt SC, Fewell JH (2019) Changing of the guard: mixed specialization and flexibility in nest defense (Tetragonisca angustula). Behav Ecol 30:1041–1049
Beshers SN, Fewell JH (2001) Models of division of labor in social insects. Annu Rev Entomol 46:413–440
Bienefeld K, Zautke F, Gupta P (2015) A novel method for undisturbed long-term observation of honey bee (Apis mellifera) behavior–illustrated by hygienic behavior towards varroa infestation. J Apic Res 54:541–547
Bonabeau E, Theraulaz G, Deneubourg JL, Aron S, Camazine S (1997) Self-organization in social insects. Trends Ecol Evol 12:188–193
Cheruiyot SK, Lattorff HMG, Kahuthia-Gathu R, Mbugi JP, Muli E (2018) Varroa-specific hygienic behavior of Apis mellifera scutellata in Kenya. Apidologie 49:439–449
Chittka L, Muller H (2009) Learning, specialization, efficiency and task allocation in social insects. Commun Integr Biol 2:151–154
Cole BJ (2020) Comparative advantage and caste evolution. Evolution 74:655–659
Danka RG, Harris JW, Dodds GE (2016) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47:483–490
Dietemann V, Pflugfelder J, Anderson D, Charrière JD, Chejanovsky N, Dainat B, de Miranda J, Delaplane K, Dillier FX, Fuch S, Gallmann P, Gauthier L, Imdorf A, Koeniger N, Kralj J, Meikle W, Pettis J, Rosenkranz P, Sammataro D, Smith D, Yañez O, Neumann P (2012) Varroa destructor: research avenues towards sustainable control. J Apic Res 51:125–132
Dornhaus A (2008) Specialization does not predict individual efficiency in an ant. PLoS Biol 6:e285
Dukas R, Visscher PK (1994) Lifetime learning by foraging honey bees. Anim Behav 48:1007–1012
Evans JD, Cook SC (2018) Genetics and physiology of Varroa mites. Curr Opin Insect Sci 26:130–135
Fries I, Camazine S, Sneyd J (1994) Population dynamics of Varroa jacobsoni: a model and a review. Bee World 75:5–28
Gordon DM (2016) From division of labor to the collective behavior of social insects. Behav Ecol Sociobiol 70:1101–1108
Gramacho KP, Spivak M (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav Ecol Sociobiol 54:472–479
Gramacho KP, Gonçalves LS, Rosenkranz P, Jong DD (1999) Influence of body fluid from pin-killed honey bee pupae on hygienic behavior. Apidologie 30:367–374
Hart AG, Anderson C, Ratnieks FL (2002) Task partitioning in leafcutting ants. Acta Ethol 5:1–11
Hurd C, Nordheim E, Jeanne R (2003) Elite workers and the colony-level pattern of labor division in the yellowjacket wasp, Vespula germanica. Behaviour 140:827–845
Jeanson R, Weidenmüller A (2014) Interindividual variability in social insects - proximate causes and ultimate consequences. Biol Rev 89:671–687
Johnson BR (2010) Task partitioning in honey bees: the roles of signals and cues in group-level coordination of action. Behav Ecol 21:1373–1379
Jongepier E, Foitzik S (2016) Fitness costs of worker specialization for ant societies. Proc R Soc B 283:20152572
Khoury DS, Myerscough MR, Barron AB (2011) A quantitative model of honey bee colony population dynamics. PLoS One 6:e18491
Kuster RD, Boncristiani HF, Rueppell O (2014) Immunogene and viral transcript dynamics during parasitic Varroa destructor mite infection of developing honey bee (Apis mellifera) pupae. J Exp Biol 217:1710–1718
Leclercq G, Pannebakker B, Gengler N, Nguyen BK, Francis F (2017) Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review. J Apic Res 56:366–375
Leclercq G, Blacquière T, Gengler N, Francis F (2018) Hygienic removal of freeze-killed brood does not predict Varroa-resistance traits in unselected stocks. J Apic Res 57:292–299
Leighton GM, Charbonneau D, Dornhaus A (2017) Task switching is associated with temporal delays in Temnothorax rugatulus ants. Behav Ecol 28:319–327
Martin SJ, Kemp D (1997) Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera) colonies. J Apic Res 36:113–123
Masterman R, Ross R, Mesce K, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Physiol A 187:441–452
McAfee A, Chapman A, Iovinella I et al (2018) A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.). Sci Rep 8:5719
Mondet F, Alaux C, Severac D, Rohmer M, Mercer AR, le Conte Y (2015) Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci Rep 5:10454
Mondet F, Kim SH, de Miranda JR, Beslay D, le Conte Y, Mercer AR (2016) Specific cues associated with honey bee social defence against Varroa destructor infested brood. Sci Rep 6:25444
Nazzi F, Della Vedova G, D’Agaro M (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35:65–70
Oddie M, Büchler R, Dahle B, Kovacic M, le Conte Y, Locke B, de Miranda JR, Mondet F, Neumann P (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8:7704
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Oxley PR, Spivak M, Oldroyd BP (2010) Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol Ecol 19:1452–1461
Page RE, Rueppell O, Amdam GV (2012) Genetics of reproduction and regulation of honeybee (Apis mellifera L.) social behavior. Annu Rev Genet 46:97–119
Palacio MA, Rodriguez E, Goncalves L, Bedascarrasbure E, Spivak M (2010) Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 41:602–612
Perez AA, Johnson BR (2019) Task repertoires of hygienic workers reveal a link between specialized necrophoric behaviors in honey bees. Behav Ecol Sociobiol 73:123
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing. URL: h//www.R-project.org/. Accessed 15 May 2018 - 30 Aug 2020
Ratnieks FL, Anderson C (1999) Task partitioning in insect societies. Ins Soc 46:95–108
Reuter GS, Spivak M (1998) A simple assay for honey bee hygienic behavior. Bee Culture 126:23–25
Rinderer TE, Harris JW, Hunt GJ, de Guzman LI (2010) Breeding for resistance to Varroa destructor in North America. Apidologie 41:409–424
Robson SK, Traniello JF (1999) Key individuals and the organisation of labor in ants. In: Detrain C, Deneubourg JL, Pasteels JM (eds) Information Processing in Social Insects. Birkhäuser Verlag, Basel, pp 239–259
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103(Suppl 1):S96–S119
Rothenbuhler WC (1964) Behaviour genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood. Am Zool 4:111–123
Rueppell O, Bachelier C, Fondrk MK, Page RE Jr (2007) Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.). Exp Gerontol 42:1020–1032
Scannapieco AC, Lanzavecchia SB, Parreño MA et al (2016) Individual precocity, temporal persistence, and task-specialization of hygienic bees from selected colonies of Apis mellifera. J Apic Sci 60:63–74
Schöning C, Gisder S, Geiselhardt S et al (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J Exp Biol 215:264–271
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press, Cambridge
Simone-Finstrom M (2017) Social immunity and the superorganism: behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World 94:21–29
Spivak M, Danka RG (2020) Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie. https://doi.org/10.1007/s13592-020-00784-z
Spivak M, Gilliam M (1998) Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa part I. hygienic behaviour and resistance to American foulbrood. Bee World 79:169–186
Sumpter DJT, Martin SJ (2004) The dynamics of virus epidemics in Varroa-infested honey bee colonies. J Anim Ecol 73:51–63
Swanson JA, Torto B, Kells SA et al (2009) Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae. J Chem Ecol 35:1108–1116
Trumbo ST, Huang Z-Y, Robinson GE (1997) Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav Ecol Sociobiol 41:151–163
Tsuruda JM, Harris JW, Bourgeois L, Danka RG, Hunt GJ (2012) High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. PLoS One 7:e48276
Wagoner KM, Rueppell O (2017) Effects of steel foundation wire on elemental content and hygienic removal of honey bee (Apis mellifera) brood. J Apic Res 56:270–277
Wagoner KM, Spivak M, Rueppell O (2018) Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). J Econ Entomol 111:2520–2530
Wagoner K, Spivak M, Hefetz A, Reams T, Rueppell O (2019) Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci Rep 9:8753
Wagoner KM, Millar JG, Schal C, Rueppell O (2020) Cuticular pheromones stimulate hygienic behavior in the honey bee (Apis mellifera). Sci Rep 10:7132
Weidenmüller A, Chen R, Meyer B (2019) Reconsidering response threshold models—short-term response patterns in thermoregulating bumblebees. Behav Ecol Sociobiol 73:112
Wilkinson D, Smith GC (2002) A model of the mite parasite, Varroa destructor, on honeybees (Apis mellifera) to investigate parameters important to mite population growth. Ecol Model 148:263–275
Wilson EO (1971) The insect societies. The Belknap Press of Harvard University Press, Cambridge
Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54:405–423
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge
Acknowledgments
We would like to warmly thank all members of the REU program and the UNCG Social Insect Lab for their encouragement and feedback. Additionally, we appreciate the time and effort of our peer reviewers to improve the quality of this manuscript.
Funding
Financial support was provided by the US National Science Foundation (DMS 1659646) and the US Department of Agriculture (National Institute for Food and Agriculture, 2017-68004-26321). The funders had no role in designing the experiments or interpreting the results.
Author information
Authors and Affiliations
Contributions
KRB, MOA, KKE, and OR designed and planned the study. Empirical data collection was primarily performed by KRB and MOA, while the simulation was performed primarily by KKE and checked by JTR. Data analysis and interpretation were performed by KRB, MOA, KKE, KMW, and OR. All authors contributed to the writing of the manuscript, with a first version drafted by KRB, MOA, and KKE and the final version completed with major revisions from KMW and OR.
Corresponding author
Ethics declarations
This work was exempt from institutional review and complies with all applicable laws of the USA. Great care was taken to not harm any bees unnecessarily.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by D. Naug
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrs, K.R., Ani, M.O., Eversman, K.K. et al. Time-accuracy trade-off and task partitioning of hygienic behavior among honey bee (Apis mellifera) workers. Behav Ecol Sociobiol 75, 12 (2021). https://doi.org/10.1007/s00265-020-02940-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02940-y