Abstract
Purpose
To determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of the combination of weekly oxaliplatin × 4, weekly irinotecan × 4 and capecitabine Monday through Friday for 4 weeks of every 6 week cycle in patients with solid tumors; to determine the pharmacokinetic profile of these agents in this combination; to observe patients for clinical anti-tumor response.
Methods
Twenty-two patients with metastatic solid tumors received oxaliplatin 60 mg/m2 weekly × 4, irinotecan beginning at a dose of 40 mg/m2 weekly × 4, and capecitabine Monday through Friday for 4 weeks of every 6 week cycle, initially at 1,000 mg twice daily (bid).
Results
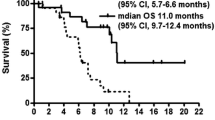
The MTD was oxaliplatin 60 mg/m2 weekly × 4, irinotecan 50 mg/m2 weekly × 4 and capecitabine 450 mg bid Monday through Friday for 4 weeks of every 6 week cycle. One of six patients at this dose level developed DLT of nausea, vomiting, and diarrhea. Among patients treated with a constant capecitabine dose of 450 mg bid, there was a higher mean AUC of 5-FU in women than in men (mean ± SD: 892 ± 287 nM h vs. 537 ± 182 nM h; Mann–Whitney two-tailed, P = 0.02). There was one complete response in a patient with gastric cancer.
Conclusion
The novel schedule of weekly oxaliplatin, weekly irinotecan, and capecitabine Monday through Friday, all administered for 4 weeks of every 6 week cycle, evaluated in this phase I trial is well-tolerated and demonstrated activity in a patient with gastric cancer.
Similar content being viewed by others
References
Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413–1418
Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352:1407–1412
Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, Itzhaki M, Metzger G, N’Daw D, Vignoud J, Abad A, Francois E, Gamelin E, Marty M, Sastre J, Seitz JF, Ychou M (1996) Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol 7:95–98
Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG (2003) Superiority of oxaliplatin and fluorouracil–leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil–leucovorin: interim results of a phase III trial. J Clin Oncol 21:2059–2069
de Gramont A, Vignoud J, Tournigand C, Louvet C, Andre T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M (1997) Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 33:214–219
Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M (1998) Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 9:105–108
Levi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declere A (1992) A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 69:893–900
Bertheault-Cvitkovic F, Jami A, Ithzaki M, Brummer PD, Brienza S, Adam R, Kunstlinger F, Bismuth H, Misset JL, Levi F (1996) Biweekly intensified ambulatory chronomodulated chemotherapy with oxaliplatin, fluorouracil, and leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 14:2950–2958
Douillard JY, Cunningham D, Roth AD, Navarro M, Karasek JP, Jandik P, Carmichael J, Alaki M, Gruia G, Awad L, Rougier P (2006) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 355:1041–1047
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehernbacher L, Moore M, Maroun JA, Ackland SP, Lockre PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. NEJM 343:905–914
Houghton JA, Cheshire PJ, Hallman JD, Lutz L, Luo X, Li Y, Houghton PJ (1996) Evaluation of irinotecan in combination with 5-fluorouracil or etoposide in xenograft models of colon adenocarcinoma and rhabdomyosarcoma. Clin Cancer Res 2:107–118
Guichard S, Hennebelle I, Bugat R, Canal P (1998) Cellular interactions of 5-fluorouracil and the camptothecin analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell line. Biochem Pharmacol 55:667–676
Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C (1997) Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 8:876–885
Giacchetti S, Zidani R, Perpoint B, Pinel MC, Faggiulo R, Focan C, Letourneau Y, Chollet P, Llory JF, Coudet B, Bertheault-Cvitkovic F, Adam R, Le Baif N, Misset JL, Bayssas M, Levi F(1997) Phase III trial of 5-fluorouracil (5-FU), folinic acid (FA), with or without oxaliplatin (OXA) in previously untreated patients (pts) with metastatic colorectal cancer (MCC) (Meeting abstract). Proc Am Soc Clin Oncol 16, abstract 805
Gramont A d, Figer A, Seymour M, Homerin M, Bail NI, Cassidy J, Boni C, Cortes-Funes H, Freyer G, Hendler D, Louvet C(1998) A randomized trial of leucovorin (LV) and 5-fluorouracil (5FU) with or without axaliplatin in advanced colorectal cancer (CRC) (Meeting abstract). Proc Am Soc Clin Oncol 17, abstract 985
Reardon JT, Vaisman A, Chaney SG, Sancar A (1999) Efficient nucleotide excision repair of cisplatin, oxaliplatin, and Bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res 59:3968–3971
Kemeny N, Tong W, Gonen M, Stockman J, Di Lauro C, Teitcher J, White P, Price C, Saltz L, Sharma S, Graham MA (2002) Phase I study of weekly oxaliplatin plus irinotecan in previously treated patients with metastatic colorectal cancer. Ann Oncol 13:1490–1496
Zeghari-Squalli N, Raymond E, Cvitkovic E, Goldwasser F (1999) Cellular pharmacology of the combination of the DNA topoisomerase I inhibitor SN-38 and the diaminocyclohexane platinum derivative oxaliplatin. Clin Cancer Res 5:1189–1196
Buechele T, Schoeber C, Kroening H, Eckart M, Lingenfelser T, Respondek M, Stier G, Balleisen L, Kallen KJ, Schmidt J, Pasold R, Spohn C, Hurtz HJ, Graubner M, Schmoll HJ (1998) Weekly high-dose (HD) 5-fluorouracil (5-FU) and folinic acid (FA) with addition of oxaliplatin (LOHP) after documented progression under high-dose infusional 5-FU/FA in patients (pts) with advanced colorectal cancer (CRC): a preliminary report (Meeting abstract). Proc Am Soc Clin Oncol 17, abstract 1106
Moehler M, Hoffmann T, Hildner K, Siebler J, Galle PR, Heike M (2002) Weekly oxaliplatin, high-dose folinic acid and 24 h-5-fluorouracil (FUFOX) as salvage therapy in metastatic colorectal cancer patients pretreated with irinotecan and folinic acid/5-fluorouracil regimens. Z Gastroenterol 40:957–964
Meta-analysis Group in Cancer (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301–308
Chansky K, Benedetti J, Macdonald JS (2005) Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 103:1165–1171
Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, Novotny PJ, Poon MA, O’Connell MJ, Loprinzi CL (2002) Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 20:1491–1498
Thomas R, Quinn M, Wilson R, Floeter M, Lehky T, Saif MW, Hamilton JM, Monahan B, Grochow L, Harold N, Schull B, Allegra C, Cliatt J, Grem J (2001) A phase I trial of capecitabine (Cape) and oxaliplatin (OHP). Proc Am Soc Clin Oncol 22, abstract 530
Erkmen K, Egorin MJ, Reyno LM, Morgan R Jr, Doroshow JH (1995) Effects of storage on the binding of carboplatin to plasma proteins. Cancer Chemother Pharmacol 35:254–256
de Bruijn P, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (1997) Determination of irinotecan (CPT-11) and its active metabolite SN-38 in human plasma by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 698:277–285
Barilero I, Gandia D, Armand JP, Mathieu-Boue A, Re M, Gouyette A, Chabot GG (1992) Simultaneous determination of the camptothecin analogue CPT-11 and its active metabolite SN-38 by high-performance liquid chromatography: application to plasma pharmacokinetic studies in cancer patients. J Chromatogr 575:275–280
Reigner B, Clive S, Cassidy J, Jodrell D, Schulz R, Goggin T, Banken L, Roos B, Utoh M, Mulligan T, Weidekamm E (1999) Influence of the antacid Maalox on the pharmacokinetics of capecitabine in cancer patients. Cancer Chemother Pharmacol 43:309–315
Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T (1998) Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16:1795–1802
Reigner B, Verweij J, Dirix L, Cassidy J, Twelves C, Allman D, Weidekamm E, Roos B, Banke L, Utoh M, Osterwalder B (1998) Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res 4:941–948
Rocci ML Jr, Jusko WJ (1983) LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed 16:203–216
Walgren RA, Meucci MA, McLeod HL (2005) Pharmacogenomic discovery approaches: will the real genes please stand up? J Clin Oncol 23:7342–7349
Goetz MP, Reid JM, Safgren SL, Mandrekar SJ, Erlichman C, Adjei AA, Goldberg RM, Grothey A, McWilliams RR, Ames MM (2007) UGT1A1*28 genotype determines the maximum tolerated dose (MTD) and pharmacokinetics (PK) of irinotecan-based chemotherapy: A phase I dose escalation trial. 2007 Gastrointestinal Cancers Symposium PROGRAM/PROCEEDINGS, abstract 235
Pitot HC, Goldberg RM, Reid JM, Sloan JA, Skaff PA, Erlichman C, Rubin J, Burch PA, Adjei AA, Alberts SA, Schaaf LJ, Elfring G, Miller LL (2000) Phase I dose-finding and pharmacokinetic trial of irinotecan hydrochloride (CPT-11) using a once-every-three-week dosing schedule for patients with advanced solid tumor malignancy. Clin Cancer Res 6:2236–2244
Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7:2182–2194
Mackean M, Planting A, Twelves C, Schellens J, Allman D, Osterwalder B, Reigner B, Griffin T, Kaye S, Verweij J (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16:2977–2985
Soepenberg O, Dumez H, Verweij J, Semiond D, de Jonge MJ, Eskens FA, Ter Steeg J, Selleslac J, Assadourian S, Sanderink GJ, Sparreboom A, van Oosterom AT (2005) Phase I and pharmacokinetic study of oral irinotecan given once daily for 5 days every 3 weeks in combination with capecitabine in patients with solid tumors. J Clin Oncol 23:889–898
Ebi H, Sigeoka Y, Saeki T, Kawada K, Igarashi T, Usubuchi N, Ueda R, Sasaki Y, Minami H (2005) Pharmacokinetic and pharmacodynamic comparison of fluoropyrimidine derivatives, capecitabine and 5′-deoxy-5-fluorouridine (5′-DFUR). Cancer Chemother Pharmacol 56:205–211
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Scheithauer W, Kornek GV, Raderer M, Schull B, Schmid K, Kovats E, Schneeweiss B, Lang F, Lenauer A, Depisch D (2003) Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21:1307–1312
Burge ME, Smith D, Topham C, Jackson DP, Anthoney DA, Halstead F, Seymour MT (2006) A phase I and II study of 2-weekly irinotecan with capecitabine in advanced gastroesophageal adenocarcinoma. Br J Cancer 94:1281–1286
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Cao S, Durrani FA, Rustum YM (2005) Synergistic antitumor activity of capecitabine in combination with irinotecan. Clin Colorectal Cancer 4:336–343
Acknowledgments
We thank the patients who participated in this trial.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by NIH grants U01 CA62502, M01-RR-00080, K12 CA76917 (SSK), P30 CA43703, U01-CA099168-01 and P30CA47904. Published in part in: Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 22, No 14S (July 15 Supplement), 2004: 2111.
Rights and permissions
About this article
Cite this article
Krishnamurthi, S.S., Brell, J.M., Hoppel, C.L. et al. Phase I clinical and pharmacokinetic study of oxaliplatin, irinotecan and capecitabine. Cancer Chemother Pharmacol 63, 441–450 (2009). https://doi.org/10.1007/s00280-008-0754-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0754-2