Abstract
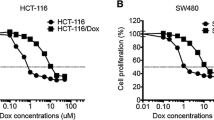
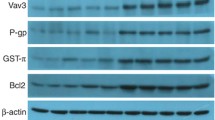
Colorectal cancer (CRC) is one of the prevalent and deadly cancers worldwide. Chemotherapy resistance is one of the most challenging problems for CRC and other cancer treatments. Recent studies indicated that increasing levels of visfatin are correlated with worse clinical prognosis of CRC patients, while the effects and mechanisms of visfatin on progression of CRC remain unclear. Our present study established doxorubicin (Dox)-resistant CRC HCT-116 and SW480 cells (named HCT-116 Dox/R and SW480 Dox/R). The expression of visfatin, while not IL-6, IL-8, or TGF-β, in CRC Dox-resistant cells was significantly greater than that in their parental cells, while knockdown of visfatin by its specific siRNAs can elevate Dox sensitivity of CRC-resistant cells. In addition, si-visfatin can significantly down regulate the expression of multidrug resistance 1 (MDR1), while not multidrug resistance-associated protein 1 or lung resistance-related protein, in both HCT-116 Dox/R and SW480 Dox/R cells. Visfatin can regulate the transcription of MDR1 via modulating its promoter activities. Si-visfatin can also decrease the activation and nuclear localization of p65, one of the most important transcription factors for the expression of MDR1. Chromatin immunoprecipitation (ChIP) indicated that si-visfatin can suppress the binding between p65 and MDR1 promoter. Collectively, our present study revealed that visfatin mediates the Dox resistance of CRC cells via up regulation of MDR1. It indicated that targeted inhibition of visfatin might be helpful for overcoming Dox resistance of CRC therapy.






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
O’Connell JB, Maggard MA, Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 96:1420–1425
Effenberger-Neidnicht K, Schobert R (2011) Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol 67:867–874
Pinzón-Daza ML, Cuellar-Saenz Y, Nualart F, Ondo-Mendez A, Del Riesgo L, Castillo-Rivera F et al (2017). Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions. J Cell Biochem 118:1868–1878
Ai S, Jia T, Ai W, Duan J, Liu Y, Chen J et al (2013) Targeted delivery of doxorubicin through conjugation with EGF receptor-binding peptide overcomes drug resistance in human colon cancer cells. Br J Pharmacol 168:1719–1735
Grolla AA, Travelli C, Genazzani AA, Sethi JK (2016) Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br J Pharmacol 173:2182–2194
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 14:1431–1437
Sampath D, Zabka TS, Misner DL, O’Brien T, Dragovich PS (2015) Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther 151:16–31
Shackelford RE, Mayhall K, Maxwell NM, Kandil E, Coppola D (2013) Nicotinamide phosphoribosyltransferase in malignancy: a review. Genes Cancer 4:447–456
Chen M, Wang Y, Li Y, Zhao L, Ye S, Wang S et al (2016) Association of plasma visfatin with risk of colorectal cancer: an observational study of Chinese patients. Asia Pac J Clin Oncol 12:e65–e74
Hufton SE, Moerkerk PT, Brandwijk R, de Bruïne AP, Arends JW, Hoogenboom HR (1999) A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett 463:77–82
Soncini D, Caffa I, Zoppoli G, Cea M, Cagnetta A, Passalacqua M et al (2014) Nicotinamide phosphoribosyltransferase promotes epithelial-to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J Biol Chem 289:34189–34204
Adya R, Tan BK, Chen J, Randeva HS (2008) Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: its role in MMP-2/9 production and activation. Diabetes Care 31:758–760
Ogawara K, Un K, Tanaka K, Higaki K, Kimura T (2009) In vivo anti-tumor effect of PEG liposomal doxorubicin (DOX) in DOX-resistant tumor-bearing mice: involvement of cytotoxic effect on vascular endothelial cells. J Control Release 133:4–10
Wei W, Chen Z-J, Zhang K-S, Yang X-L, Wu Y-M, Chen X-H et al (2014) The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis 5:e1428
Sui H, Pan S-F, Feng Y, Jin B-H, Liu X, Zhou L-H et al (2014) Zuo Jin Wan reverses P-gp-mediated drug-resistance by inhibiting activation of the PI3 K/Akt/NF-κB pathway. BMC Complement Altern Med 14:279
Gupta SV, Sass EJ, Davis ME, Edwards RB, Lozanski G, Heerema NA et al (2011) Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J 13:357
Shen H, Xu W, Chen Q, Wu Z, Tang H, Wang F (2010) Tetrandrine prevents acquired drug resistance of K562 cells through inhibition of mdr1 gene transcription. J Cancer Res Clin Oncol 136:659–665
West NR, McCuaig S, Franchini F, Powrie F (2015) Emerging cytokine networks in colorectal cancer. Nat Rev Immunol 15:615–629
Celestino AT, Levy D, Maria Ruiz JL, Bydlowski SP (2015) ABCB1, ABCC1, and LRP gene expressions are altered by LDL, HDL, and serum deprivation in a human doxorubicin-resistant uterine sarcoma cell line. Biochem Biophys Res Commun 457:664–668
Wang L, Meng Q, Wang C, Liu Q, Peng J, Huo X et al (2013) Dioscin restores the activity of the anticancer agent adriamycin in multidrug-resistant human leukemia K562/adriamycin cells by down-regulating MDR1 via a mechanism involving NF-κB signaling inhibition. J Nat Prod 76:909–914
Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S et al (2010) Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci 101:1286–1291
Yang J, Zhang K, Song H, Wu M, Li J, Yong Z et al (2016) Visfatin is involved in promotion of colorectal carcinoma malignancy through an inducing EMT mechanism. Oncotarget 7:32306–32317
Feng J, Yan P-F, Zhao H, Zhang F-C, Zhao W-H, Feng M (2016) Inhibitor of nicotinamide phosphoribosyltransferase sensitizes glioblastoma cells to temozolomide via activating ROS/JNK signaling pathway. BioMed Res Int. doi:10.1155/2016/1450843
Sims JT, Ganguly SS, Bennett H, Friend JW, Tepe J, Plattner R (2013) Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS One 8:e55509
Jieyu H, Chao T, Mengjun L, Shalong W, Xiaomei G, Jianfeng L et al (2012) Nampt/Visfatin/PBEF: a functionally multi-faceted protein with a pivotal role in malignant tumors. Curr Pharm Des 18:6123–6132
Wang G-J, Shen N-J, Cheng L, Fang Y, Huang H, Li K-H (2016) Visfatin triggers the in vitro migration of osteosarcoma cells via activation of NF-κB/IL-6 signals. Eur J Pharmacol 791:322–330
Kuo MT, Liu Z, Wei Y, Lin-Lee Y, Tatebe S, Mills GB et al (2002) Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 21:1945–1954
Acknowledgements
This research was supported by the National Natural Science Fund from the National Natural Science Foundation of China (Grant no. 81672427).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. There is no human/animal participant in the present study.
Rights and permissions
About this article
Cite this article
Yan, X., Zhao, J. & Zhang, R. Visfatin mediates doxorubicin resistance in human colorectal cancer cells via up regulation of multidrug resistance 1 (MDR1). Cancer Chemother Pharmacol 80, 395–403 (2017). https://doi.org/10.1007/s00280-017-3365-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3365-y