Abstract
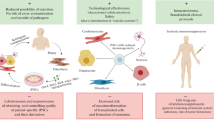
The potential to develop into any tissue makes pluripotent stem cells (PSCs) one of the most promising sources for cellular therapeutics. However, numerous hurdles exist to their clinical applications, three of the most concerning include the inability to separate therapeutic population from heterogeneously differentiated cultures, the risk of teratoma formation from residual pluripotent cells, and immunologic rejection of engrafted cells. The recent development of induced PSCs has been proposed as a solution to the histocompatibility barrier. Theoretically, creation of patient-specific induced PSC lines would exhibit a complete histocompatibility antigen match. However, regardless of the PSC source, in vitro propagation and nonphysiologic differentiation may result in other, likely less powerful, mechanisms of immune rejection. In light of recent progress towards clinical application, this review focuses on two such potential immunologic mechanisms applicable to isogenic PSC derivates: namely, the immunogenicity of aberrant antigens resulting from long-term in vitro maintenance and alterations in immunologic properties due to rapid in vitro differentiation. These issues will be considered with attention to their relation to effector cells in the adult immune system. In addition, we highlight immunosuppressive approaches that could potentially address the immunogenicity of these proposed mechanisms.
Similar content being viewed by others
References
Nankivell BJ, Alexander SI (2010) Rejection of the kidney allograft. N Engl J Med 363:1451–1462
Drukker M, Katz G, Urbach A, Schuldiner M, Markel G et al (2002) Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA 99:9864–9869
Hwang WS, Ryu YJ, Park JH, Park ES, Lee EG et al (2004) Evidence of a pluripotent human embryonic stem cell line derived from a cloned blastocyst. Science 303:1669–1674
McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE et al (2000) Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature 405:1066–1069
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Utermohlen O, Baschuk N, Abdullah Z, Engelmann A, Siebolts U et al (2009) Immunologic hurdles of therapeutic stem cell transplantation. Biol Chem 390:977–983
Drukker M (2004) Immunogenicity of human embryonic stem cells: can we achieve tolerance? Springer Semin Immunopathol 26:201–213
Drukker M, Benvenisty N (2004) The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol 22:136–141
Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S et al (2007) N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells 25:197–202
Martin MJ, Muotri A, Gage F, Varki A (2005) Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 11:228–232
Chung TL, Turner JP, Thaker N, Kolle G, Cooper-White JJ et al (2010) Ascorbate promotes epigenetic activation of CD30 in human embryonic stem cells. Stem Cells 28(10):1782–1793
Draper JS, Smith K, Gokhale P, Moore HD, Maltby E et al (2004) Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22:53–54
Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C et al (2008) Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol 26:1361–1363
Fernandez N, Cooper J, Sprinks M, AbdElrahman M, Fiszer D et al (1999) A critical review of the role of the major histocompatibility complex in fertilization, preimplantation development and feto-maternal interactions. Hum Reprod Update 5:234–248
Gardner JM, Fletcher AL, Anderson MS, Turley SJ (2009) AIRE in the thymus and beyond. Curr Opin Immunol 21:582–589
Lei T, Jacob S, Ajil-Zaraa I, Dubuisson JB, Irion O et al (2007) Xeno-free derivation and culture of human embryonic stem cells: current status, problems and challenges. Cell Res 17:682–688
Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N et al (2003) Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA 100:12045–12050
Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E et al (1998) A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA 95:11751–11756
Cerdan C, Bendall SC, Wang L, Stewart M, Werbowetski T et al (2006) Complement targeting of nonhuman sialic acid does not mediate cell death of human embryonic stem cells. Nat Med 12:1113–1114, author reply 1115
Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER et al (2006) Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24:185–187
Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD (2006) Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol 38:1063–1075
Xu C, Inokuma MS, Denham J, Golds K, Kundu P et al (2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19:971–974
Prowse AB, Doran MR, Cooper-White JJ, Chong F, Munro TP et al (2010) Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials 31:8281–8288
Rajala K, Hakala H, Panula S, Aivio S, Pihlajamaki H et al (2007) Testing of nine different xeno-free culture media for human embryonic stem cell cultures. Hum Reprod 22:1231–1238
Lefort N, Feyeux M, Bas C, Feraud O, Bennaceur-Griscelli A et al (2008) Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat Biotechnol 26:1364–1366
Werbowetski-Ogilvie TE, Bosse M, Stewart M, Schnerch A, Ramos-Mejia V et al (2009) Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol 27:91–97
Herszfeld D, Wolvetang E, Langton-Bunker E, Chung TL, Filipczyk AA et al (2006) CD30 is a survival factor and a biomarker for transformed human pluripotent stem cells. Nat Biotechnol 24:351–357
Al-Shamkhani A (2004) The role of CD30 in the pathogenesis of haematopoietic malignancies. Curr Opin Pharmacol 4:355–359
Kim K, Doi A, Wen B, Ng K, Zhao R et al (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467:285–290
Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW et al (2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 25:803–816
Moore JC, Sadowy S, Alikani M, Toro-Ramos AJ, Swerdel MR et al (2010) A high-resolution molecular-based panel of assays for identification and characterization of human embryonic stem cell lines. Stem Cell Res 4:92–106
Akopian V, Andrews PW, Beil S, Benvenisty N, Brehm J et al (2010) Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim 46:247–258
International Stem Cell Banking Initiative (2009) Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev 5:301–314
Suarez-Alvarez B, Rodriguez RM, Calvanese V, Blanco-Gelaz MA, Suhr ST et al (2010) Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS ONE 5:e10192
Sprinks MT, Sellens MH, Dealtry GB, Fernandez N (1993) Preimplantation mouse embryos express Mhc class I genes before the first cleavage division. Immunogenetics 38:35–40
Cooper JC, Fernandez N, Joly E, Dealtry GB (1998) Regulation of major histocompatibility complex and TAP gene products in preimplantation mouse stage embryos. Am J Reprod Immunol 40:165–171
Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E et al (2006) Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24:221–229
Lampton PW, Crooker RJ, Newmark JA, Warner CM (2008) Expression of major histocompatibility complex class I proteins and their antigen processing chaperones in mouse embryonic stem cells from fertilized and parthenogenetic embryos. Tissue Antigens 72:448–457
Mammolenti M, Gajavelli S, Tsoulfas P, Levy R (2004) Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells 22:1101–1110
Wu DC, Boyd AS, Wood KJ (2008) Embryonic stem cells and their differentiated derivatives have a fragile immune privilege but still represent novel targets of immune attack. Stem Cells 26:1939–1950
Boyd AS, Wood KJ (2009) Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters. Transplantation 87:1300–1304
Dressel R, Guan K, Nolte J, Elsner L, Monecke S et al (2009) Multipotent adult germ-line stem cells, like other pluripotent stem cells, can be killed by cytotoxic T lymphocytes despite low expression of major histocompatibility complex class I molecules. Biol Direct 4:31
Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H et al (2007) Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci USA 104:20920–20925
Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K et al (2008) Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA 105:12991–12996
Yen BL, Chang CJ, Liu KJ, Chen YC, Hu HI et al (2009) Brief report—human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 27:451–456
Lui KO, Boyd AS, Cobbold SP, Waldmann H, Fairchild PJ (2010) A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across an major histocompatibility complex barrier. Stem Cells 28(10):1905–1914
Grinnemo KH, Genead R, Kumagai-Braesch M, Andersson A, Danielsson C et al (2008) Costimulation blockade induces tolerance to HESC transplanted to the testis and induces regulatory T-cells to HESC transplanted into the heart. Stem Cells 26:1850–1857
Aluvihare VR, Kallikourdis M, Betz AG (2004) Regulatory T-cells mediate maternal tolerance to the fetus. Nat Immunol 5:266–271
Trowsdale J, Betz AG (2006) Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 7:241–246
Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A et al (2004) Decidual and peripheral blood CD4 + CD25+ regulatory T-cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10:347–353
Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T et al (2010) Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci USA 107:13022–13027
Nitta T, Murata S, Ueno T, Tanaka K, Takahama Y (2008) Thymic microenvironments for T-cell repertoire formation. Adv Immunol 99:59–94
Haynes BF, Heinly CS (1995) Early human T-cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med 181:1445–1458
Res P, Spits H (1999) Developmental stages in the human thymus. Semin Immunol 11:39–46
Nagano K, Yoshida Y, Isobe T (2008) Cell surface biomarkers of embryonic stem cells. Proteomics 8:4025–4035
Schopperle WM, DeWolf WC (2007) The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells 25:723–730
Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S et al (2007) The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells 25:54–62
Li Y, Zeng H, Xu RH, Liu B, Li Z (2009) Vaccination with human pluripotent stem cells generates a broad spectrum of immunological and clinical responses against colon cancer. Stem Cells 27:3103–3111
Siegel S, Wagner A, Kabelitz D, Marget M, Coggin J Jr et al (2003) Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 102:4416–4423
Zelle-Rieser C, Barsoum AL, Sallusto F, Ramoner R, Rohrer JW et al (2001) Expression and immunogenicity of oncofetal antigen-immature laminin receptor in human renal cell carcinoma. J Urol 165:1705–1709
Tchabo NE, Mhawech-Fauceglia P, Caballero OL, Villella J, Beck AF et al (2009) Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun 9:6
Dong W, Du J, Shen H, Gao D, Li Z et al (2010) Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load. Cancer Immunol Immunother 59:1697–1705
Frenzel LP, Abdullah Z, Kriegeskorte AK, Dieterich R, Lange N et al (2009) Role of natural-killer group 2 member D ligands and intercellular adhesion molecule 1 in natural killer cell-mediated lysis of murine embryonic stem cells and embryonic stem cell-derived cardiomyocytes. Stem Cells 27:307–316
Preynat-Seauve O, de Rham C, Tirefort D, Ferrari-Lacraz S, Krause KH et al (2009) Neural progenitors derived from human embryonic stem cells are targeted by allogeneic T and natural killer cells. J Cell Mol Med 13:3556–3569
Dressel R, Schindehutte J, Kuhlmann T, Elsner L, Novota P et al (2008) The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS ONE 3:e2622
Dressel R, Nolte J, Elsner L, Novota P, Guan K et al (2010) Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J 24(7):2164–2177
Magliocca JF, Held IK, Odorico JS (2006) Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression. Stem Cells Dev 15:707–717
Yokoyama WM, Kim S (2006) How do natural killer cells find self to achieve tolerance? Immunity 24:249–257
Orr MT, Lanier LL (2010) Natural killer cell education and tolerance. Cell 142:847–856
Jonsson AH, Yokoyama WM (2009) Natural killer cell tolerance licensing and other mechanisms. Adv Immunol 101:27–79
Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A et al (2004) Human embryonic stem cells possess immune-privileged properties. Stem Cells 22:448–456
Guller S, LaChapelle L (1999) The role of placental Fas ligand in maintaining immune privilege at maternal–fetal interfaces. Semin Reprod Endocrinol 17:39–44
Yachimovich-Cohen N, Even-Ram S, Shufaro Y, Rachmilewitz J, Reubinoff B (2010) Human embryonic stem cells suppress T-cell responses via arginase I-dependent mechanism. J Immunol 184:1300–1308
Koch CA, Geraldes P, Platt JL (2008) Immunosuppression by embryonic stem cells. Stem Cells 26:89–98
Trigona WL, Porter CM, Horvath-Arcidiacono JA, Majumdar AS, Bloom ET (2007) Could heme-oxygenase-1 have a role in modulating the recipient immune response to embryonic stem cells? Antioxid Redox Signal 9:751–756
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5:641–654
Ishikawa T, Harada T, Koi H, Kubota T, Azuma H et al (2007) Identification of arginase in human placental villi. Placenta 28:133–138
Kropf P, Baud D, Marshall SE, Munder M, Mosley A et al (2007) Arginase activity mediates reversible T-cell hyporesponsiveness in human pregnancy. Eur J Immunol 37:935–945
Fandrich F, Lin X, Chai GX, Schulze M, Ganten D et al (2002) Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med 8:171–178
Fabricius D, Bonde S, Zavazava N (2005) Induction of stable mixed chimerism by embryonic stem cells requires functional Fas/FasL engagement. Transplantation 79:1040–1044
Bonde S, Zavazava N (2006) Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells 24:2192–2201
Hunt JS, Vassmer D, Ferguson TA, Miller L (1997) Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol 158:4122–4128
Brunlid G, Pruszak J, Holmes B, Isacson O, Sonntag KC (2007) Immature and neurally differentiated mouse embryonic stem cells do not express a functional Fas/Fas ligand system. Stem Cells 25:2551–2558
Kelly CM, Precious SV, Scherf C, Penketh R, Amso NN et al (2009) Neonatal desensitization allows long-term survival of neural xenotransplants without immunosuppression. Nat Methods 6:271–273
Matzinger P, Kamala T (2011) Tissue-based class control: the other side of tolerance. Nat Rev Immunol 11:221–230
Ferguson TA, Griffith TS (2006) A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev 213:228–238
Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:4
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Immunopathology of Pluripotent Stem Cell Transplantation [33:6]
Rights and permissions
About this article
Cite this article
Tang, C., Drukker, M. Potential barriers to therapeutics utilizing pluripotent cell derivatives: intrinsic immunogenicity of in vitro maintained and matured populations. Semin Immunopathol 33, 563–572 (2011). https://doi.org/10.1007/s00281-011-0269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-011-0269-5