Abstract
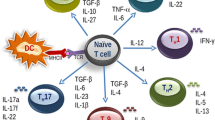
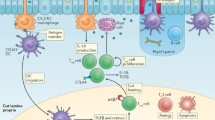
Food allergy is a harmful immune reaction driven by uncontrolled type 2 immune responses. Considerable evidence demonstrates the key roles of mast cells, IgE, and TH2 cytokines in mediating food allergy. However, this evidence provides limited insight into why only some, rather than all, food allergic individuals are prone to develop life-threatening anaphylaxis. Clinical observations suggest that patients sensitized to food through the skin early in life may later develop severe food allergies. Aberrant epidermal thymic stromal lymphopoietin and interleukin (IL) 33 production and genetic predisposition can initiate an allergic immune response mediated by dendritic cells and CD4+TH2 cells in inflamed skin. After allergic sensitization, intestinal IL-25 and food ingestion enhance concerted interactions between type 2 innate lymphoid cells (ILC2s) and CD4+TH2 cells, which perpetuate allergic reactions from the skin to the gut. IL-4 and cross-linking of antigen/IgE/FcεR complexes induce emigrated mast cell progenitors to develop into the multi-functional IL-9-producing mucosal mast cells, which produce prodigious amounts of IL-9 and mast cell mediators to drive intestinal mastocytosis in an autocrine loop. ILC2s and TH9 cells may also serve as alternative cellular sources of IL-9 to augment the amplification of intestinal mastocytosis, which is the key cellular checkpoint in developing systemic anaphylaxis. These findings provide a plausible view of how food allergy develops and progresses in a stepwise manner and that atopic signals, dietary allergen ingestion, and inflammatory cues are fundamental in promoting life-threatening anaphylaxis. This information will aid in improving diagnosis and developing more effective therapies for food allergy-triggered anaphylaxis.

Similar content being viewed by others
References
Gupta RS et al (2011) The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 128(1):e9–17
Sicherer SH, Sampson HA (2009) Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 60:261–277
Burbank AJ et al (2016) Oral immunotherapy for food allergy. Immunol Allergy Clin N Am 36(1):55–69
Wood RA (2016) Food allergen immunotherapy: current status and prospects for the future. J Allergy Clin Immunol. 137(4):973–982
Burks AW, Sampson HA (1999) Anaphylaxis and food allergy. Clinical reviews in allergy & immunology 17(3):339–360
Sicherer SH, Sampson HA (2000) Peanut and tree nut allergy. Curr Opin Pediatr 12(6):567–573
Sicherer SH, Sampson HA (1999) Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 104(3 Pt 2):S114–S122
Burks AW et al (2012) Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367(3):233–243
Du Toit G et al (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372(9):803–813
Vazquez-Ortiz M, Turner PJ (2016) Improving the safety of oral immunotherapy for food allergy. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 27(2):117–125
Sampson HA (1999) Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 103(5 Pt 1):717–728
Hadis U et al (2011) Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34(2):237–246
Noval Rivas M et al (2015) Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 42(3):512–523
Schulz O et al (2009) Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206(13):3101–3114
Coombes JL et al (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204(8):1757–1764
Curotto de Lafaille MA et al (2008) Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29(1):114–126
Josefowicz SZ et al (2012) Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482(7385):395–399
Karlsson MR et al (2004) Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med 199(12):1679–1688
Palmer DJ et al (2013) Early regular egg exposure in infants with eczema: a randomized controlled trial. The Journal of allergy and clinical immunology. 132(2):387–92 e1
Hill DJ et al (2008) Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: an international study. Clin Exp Allergy 38(1):161–168
Lack G et al (2003) Factors associated with the development of peanut allergy in childhood. N Engl J Med 348(11):977–985
Koplin JJ et al (2015) Cohort profile: the HealthNuts study: population prevalence and environmental/genetic predictors of food allergy. Int J Epidemiol 44(4):1161–1171
Noti M et al (2014) Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol 133(5):1390-9–9 e1-6
Mathias CB et al (2011) IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol 127(3):795–805 e1-6
Chen CY et al (2015) Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity 43(4):788–802
Tordesillas L et al (2014) Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 124(11):4965–4975
Haas H et al (1999) Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur J Immunol 29(3):918–927
Phillips C et al (2003) Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol 73(1):165–171
Reese TA et al (2007) Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447(7140):92–96
Liu YJ et al (2007) TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. AnnuRevImmunol 25:193–219
Friend SL et al (1994) A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 22(3):321–328
Watanabe N et al (2005) Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436(7054):1181–1185
Ying S et al (2005) Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. JImmunol. 174(12):8183–8190
Soumelis V et al (2002) Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. NatImmunol 3(7):673–680
Li M et al (2006) Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. ProcNatlAcadSciUSA. 103(31):11736–11741
Oyoshi MK et al (2010) Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 126(5):976–84 e1-5
Lee HC, Ziegler SF (2007) Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. ProcNatlAcadSciUSA 104(3):914–919
Wang YH et al (2006) Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity 24(6):827–838
Wang Q et al (2015) Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol. 135(3):781–91 e3
Siracusa MC et al (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477(7363):229–233
Han H et al (2014) Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest 124(12):5442–5452
Bonnelykke K et al (2014) A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 46(1):51–55
Moffatt MF et al (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363(13):1211–1221
Martin NT, Martin MU (2016) Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 17(2):122–131
Hardman C, Ogg G (2016) Interleukin-33, friend and foe in type-2 immune responses. Curr Opin Immunol 42:16–24
Galand C et al (2016) IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. doi:10.1016/j.jaci.2016.03.056
Fort MM et al (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15(6):985–995
Pan G et al (2001) Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. JImmunol 167(11):6559–6567
Kim MR et al (2002) Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood 100(7):2330–2340
Zaph C et al (2008) Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 205(10):2191–2198
Caruso R et al (2009) Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology 136(7):2270–2279
von Moltke J et al (2016) Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529(7585):221–225
Fallon PG et al (2006) Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203(4):1105–1116
Lee JB et al (2016) IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol 137(4):1216–25 e1-5
Oliphant CJ et al (2014) MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41(2):283–295
Renauld JC et al (1990) Human P40/IL-9. Expression in activated CD4+ T cells, genomic organization, and comparison with the mouse gene. J Immunol 144(11):4235–4241
Louahed J et al (2000) Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol 22(6):649–656
McLane MP et al (1998) Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am J Respir Cell Mol Biol 19(5):713–720
Temann UA et al (1998) Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med 188(7):1307–1320
Kung TT et al (2001) Effect of anti-mIL-9 antibody on the development of pulmonary inflammation and airway hyperresponsiveness in allergic mice. Am J Respir Cell Mol Biol 25(5):600–605
McMillan SJ et al (2002) The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med 195(1):51–57
Faulkner H et al (1998) Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun 66(8):3832–3840
Angkasekwinai P et al (2013) Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect Immun 81(10):3731–3741
Townsend JM et al (2000) IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13(4):573–583
Forbes EE et al (2008) IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med 205(4):897–913
Osterfeld H et al (2010) Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 125(2):469–76 e2
Dardalhon V et al (2008) IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 9(12):1347–1355
Veldhoen M et al (2008) Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 9(12):1341–1346
Chang HC et al (2010) The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 11(6):527–534
Staudt V et al (2010) Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33(2):192–202
Angkasekwinai P et al (2007) Interleukin 25 promotes the initiation of proallergic type 2 responses. JExpMed 204(7):1509–1517
Wang YH et al (2007) IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204(8):1837–1847
Angkasekwinai P et al (2010) Regulation of IL-9 expression by IL-25 signaling. Nat Immunol 11(3):250–256
Yao W et al (2013) Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity 38(2):360–372
Jones CP et al (2012) Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 129(4):1000–10 e3
Sehra S et al (2015) TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 136(2):433–40 e1
Licona-Limon P et al (2013) Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39(4):744–757
Purwar R et al (2012) Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 18(8):1248–1253
Brough HA et al (2014) IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J Allergy Clin Immunol. 134(6):1329–38 e10
Stone SF et al (2009) Elevated serum cytokines during human anaphylaxis: identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol 124(4):786–92 e4
Perdue MH et al (1991) Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest 87(2):687–693
Radauer C et al (2008) Allergens are distributed into few protein families and possess a restricted number of biochemical functions. The Journal of allergy and clinical immunology 121(4):847–52 e7
Wilhelm C et al (2011) An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol 12(11):1071–1077
Wood RA et al (2016) A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 137(4):1103–10 e1-11
Acknowledgements
We thank S. Hottinger for editorial assistance. This work is supported by the National Institutes of Health (R01 AI090129-1, R01 AI112626-01, and 2U19 AI070235-11), Department of Defense (W81XWH-15-1-0517), and the Digestive Health Center of Cincinnati Children’s Hospital Medical Center (P30 DK078392).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on Th9 Cells in Immunity and Immunopathological Diseases - Guest Editors: Mark Kaplan and Markus Neurath
Rights and permissions
About this article
Cite this article
Shik, D., Tomar, S., Lee, JB. et al. IL-9-producing cells in the development of IgE-mediated food allergy. Semin Immunopathol 39, 69–77 (2017). https://doi.org/10.1007/s00281-016-0605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-016-0605-x