Abstract
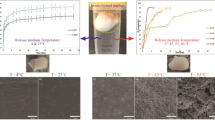
This work investigates the degradation of PLGA implants in an aqueous medium maintained at physiological pH ≈ 7.4. Two limiting systems are also investigated, which involve the degradation of PLGA microspheres in two different media characterized by: (i) a non-regulated pH, for emulating the autocatalyzed degradation in the implant core; and (ii) a regulated physiological pH, for emulating the uncatalyzed degradation at the implant surface. The degradation experiments were carried out along 40–50 days, and samples withdrawn during this period were characterized by gravimetry, electronic microscopy, and size exclusion chromatography. Experimental results suggest that PLGA implants are degraded according to a time-variant spatial pattern, which depends on the pH of the surrounding medium. Initially, the implants suffered a typically bulk erosion process, governed by the acidification of the implant core; and after breakage of the implant wall, the regulated physiological pH induces a surface erosion process. The two auxiliary microsphere-based experiments were useful to elucidate the degradation phenomena occurring in the PLGA implants. The evolution of the mass loss and the weight-average molecular weight along the degradation can be successfully predicted by simple mathematical models based on first-order kinetics.









Similar content being viewed by others
References
Rajeev AJ (2000) The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21(23):2475–2490. doi:10.1016/s0142-9612(00)00115-0
Fredenberg S, Wahlgren M, Reslow M, Axelsson A (2011) The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm 415(1–2):34–52. doi:10.1016/j.ijpharm.2011.05.049
Hatefi A, Amsden B (2002) Biodegradable injectable in situ forming drug delivery systems. J Control Release 80(1–3):9–28. doi:10.1016/s0168-3659(02)00008-1
Packhaeuser CB, Schnieders J, Oster CG, Kissel T (2004) In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm 58(2):445–455. doi:10.1016/j.ejpb.2004.03.003
Jain GK, Pathan SA, Akhter S, Ahmad N, Jain N, Talegaonkar S, Khar RK, Ahmad FJ (2010) Mechanistic study of hydrolytic erosion and drug release behaviour of PLGA nanoparticles: influence of chitosan. Polym Degrad Stabil 95(12):2360–2366. doi:10.1016/j.polymdegradstab.2010.08.015
Göpferich A (1997) Mechanisms of polymer degradation and elimination. In: Domb AJ, Kost J, Wiseman DM (eds) Handbook of biodegradable polymers. Harwood Academic Publishers, Amsterdam, pp 451–471
Göpferich A (1996) Mechanisms of polymer degradation and erosion. Biomaterials 17(2):103–114. doi:10.1016/0142-9612(96)85755-3
Li SM, Garreau H, Vert M (1990) Structure-property relationships in the case of the degradation of massive aliphatic poly-(α-hydroxy acids) in aqueous media. J Mater Sci Mater Med 1(3):123–130. doi:10.1007/bf00700871
Tamada JA, Langer R (1993) Erosion kinetics of hydrolytically degradable polymers. Proc Natl Acad Sci 90(2):552–556
Yoshioka T, Kawazoe N, Tateishi T, Chen G (2008) In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials 29(24–25):3438–3443. doi:10.1016/j.biomaterials.2008.04.011
Grizzi I, Garreau H, Li S, Vert M (1995) Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 16(4):305–311
Chen Y, Zhou S, Li Q (2011) Mathematical modeling of degradation for bulk-erosive polymers: applications in tissue engineering scaffolds and drug delivery systems. Acta Biomater 7(3):1140–1149
Krebs MD, Sutter KA, Lin ASP, Guldberg RE, Alsberg E (2009) Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering. Acta Biomater 5(8):2847–2859. doi:10.1016/j.actbio.2009.04.035
Dånmark S, Finne-Wistrand A, Schander K, Hakkarainen M, Arvidson K, Mustafa K, Albertsson AC (2011) In vitro and in vivo degradation profile of aliphatic polyesters subjected to electron beam sterilization. Acta Biomater 7(5):2035–2046. doi:10.1016/j.actbio.2011.02.011
Körber M (2010) PLGA erosion: solubility- or diffusion-controlled? Pharm Res 27(11):2414–2420
Schliecker G, Schmidt C, Fuchs S, Wombacher R, Kissel T (2003) Hydrolytic degradation of poly(lactide-co-glycolide) films: effect of oligomers on degradation rate and crystallinity. Int J Pharm 266(1–2):39–49. doi:10.1016/s0378-5173(03)00379-x
Wang L, Venkatraman S, Kleiner L (2004) Drug release from injectable depots: two different in vitro mechanisms. J Control Release 99(2):207–216. doi:10.1016/j.jconrel.2004.06.021
Graham PD, Brodbeck KJ, McHugh AJ (1999) Phase inversion dynamics of PLGA solutions related to drug delivery. J Control Release 58(2):233–245
Dunn RL, English, James P, Cowsar, Donald R, Vanderbilt, David P (1990) Biodegradable in situ forming implants and methods of producing the same. US Patent Patent 5990194
Sah H (1997) Microencapsulation techniques using ethyl acetate as a dispersed solvent: effects of its extraction rate on the characteristics of PLGA microspheres. J Control Release 47(3):233–245. doi:10.1016/S0168-3659(97)01647-7
Mohd-Adnan A-F, Nishida H, Shirai Y (2008) Evaluation of kinetics parameters for poly(l-lactic acid) hydrolysis under high-pressure steam. Polym Degrad Stabil 93(6):1053–1058. doi:10.1016/j.polymdegradstab.2008.03.022
Sadao Mori HGB (1999) Size exclusion chromatography. In: Springer Laboratory, Springer Lab Manuals Series, Laboratory Series, 1st edn. Springer, Germany
Meira GR, Vega JR, Yossen MM (2004) Gel permeation and size exclusion chromatography. In: Cazes J (ed) Ewing’s analytical instrumentation handbook, 3rd edn. Marcel Dekker Inc., New York, pp 827–869
Kenley RA, Lee MO, Mahoney TR, Sanders LM (1987) Poly(lactide-co-glycolide) decomposition kinetics in vivo and in vitro. Macromolecules 20(10):2398–2403
Chiu LK, Chiu WJ, Cheng YL (1995) Effects of polymer degradation on drug released-mechanistic study of morphology and transport properties in 50:50 poly(dl-lactide-co-glycolide). Int J Pharm 126(1–2):169–178
Göpferich A (1997) Polymer bulk erosion. Macromolecules 30(9):2598–2604
Vey E, Rodger C, Booth J, Claybourn M, Miller AF, Saiani A (2011) Degradation kinetics of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution as revealed by infrared and Raman spectroscopies. Polym Degrad Stabil 96(10):1882–1889. doi:10.1016/j.polymdegradstab.2011.07.011
Mobedi H, Nekoomanesh M, Orafaei H, Mivehchi H (2006) Studying the degradation of poly(l-lactide) in presence of magnesium hydroxide. Iran Polym J 15(1):31–39
Zolnik BS, Burgess DJ (2007) Effect of acidic pH on PLGA microsphere degradation and release. J Control Release 122(3):338–344. doi:10.1016/j.jconrel.2007.05.034
Park TG (1994) Degradation of poly(d, l-lactic acid) microspheres: effect of molecular weight. J Control Release 30(2):161–173. doi:10.1016/0168-3659(94)90263-1
Dong WY, Körber M, López Esguerra V, Bodmeier R (2006) Stability of poly(d, l-lactide-co-glycolide) and leuprolide acetate in in situ forming drug delivery systems. J Control Release 115(2):158–167. doi:10.1016/j.jconrel.2006.07.013
Gasparini G, Holdich RG, Kosvintsev SR (2010) PLGA particle production for water-soluble drug encapsulation: degradation and release behaviour. Colloid Surf B 75(2):557–564
Blasi P, D’Souza SS, Selmin F, DeLuca PP (2005) Plasticizing effect of water on poly(lactide-co-glycolide). J Control Release 108(1):1–9
Yoo JY, Kim JM, Seo KS, Jeong YK, Lee HB, Khang G (2005) Characterization of degradation behavior for PLGA in various pH condition by simple liquid chromatography method. Biomed Mater Eng 15(4):279–288
Lam CXF, Savalani MM, Teoh S-H, Hutmacher DW (2008) Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater 3(3):034108
Acknowledgments
In memory of Dr. Ricardo J.A. Grau and Dra. María Inés Cabrera, who passed away while this paper was in preparation. The authors are grateful for the financial support received from the following Argentine institutions: Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Universidad Nacional del Litoral (UNL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boimvaser, S., Mariano, R.N., Turino, L.N. et al. In vitro bulk/surface erosion pattern of PLGA implant in physiological conditions: a study based on auxiliary microsphere systems. Polym. Bull. 73, 209–227 (2016). https://doi.org/10.1007/s00289-015-1481-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1481-6