Abstract
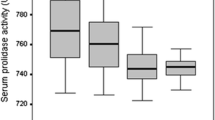
The role of paraoxonase (PON) and arylesterase (ARE) in the pathogenesis of inflammatory arthritis has been investigated, and their serum levels have been evaluated, but clinical study concerning PON1 and ARE and ankylosing spondylitis (AS) is little reported in literature. The aim of this study was to investigate PON1 and ARE activities in AS in comparison with healthy persons and their relation with the disease activity parameters. 35 AS patients and 35 healthy controls (matched for age and sex) participated. Disease activity of AS patients was assessed clinically according to the Bath AS disease activity index, and AS functional impairment was assessed using the Bath AS Functional Index. Serum samples were collected from all subjects to evaluate serum PON1, ARE activities, and lipid profile. The mean serum triglycerides, total cholesterol, and low density lipoprotein (LDL) were significantly higher in the AS patients than in controls, while the high-density lipoprotein (HDL) is significantly lower in the AS patients than controls. Serum PON1 and ARE activities were significantly lower in AS patients than in controls, while malondialdehyde (MDA) was significantly higher. AS patients with active disease had significantly higher serum triglycerides, total cholesterol, LDL and MDA while lower HDL, PON1 and ARE than those with no active AS. Decrease in the PON1/ARE activity leading to generation of oxidative stress may play an important role in the pathogenesis of AS. Moreover, it seems that activity of PON1/ARE in patients with AS is strictly correlated with the activity of the inflammatory process.






Similar content being viewed by others
References
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724
Gambhir JK, Lali P, Jain AK (1997) Correlation between blood antioxidant levels and lipid peroxidation in rheumatoid arthritis. Clin Biochem 30:351–355
Halliwell B (1996) Antioxidants in human health and disease. Annu Rev Nutr 16:33–50
Mazzetti I, Grigolo B, Pulsatelli L et al (2001) Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci (Lond) 101:593–599
Hadjigogos K (2003) The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med 45:7–13
Mackness MI, Mackness B, Durrington PN, Connelly PW et al (1996) Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol 7:69–76
Aviram M, Rosenblat M, Bisgaier CL, Newton RS et al (1998) Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 101:1581–1590
Feingold KR, Memon RA, Moser AH, Grunfeld C (1998) Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 139:307–315
Mackness B, Mackness MI, Arrol S, Turkie W et al (1998) Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett 423:57–60
Rozenberg O, Rosenblat M, Coleman R, Shih DM et al (2003) Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med 34:774–784
Karakaya A, Suzen S, Sardas S, Karakaya AE, Vural N (1991) Analysis of the serum paraoxonase/arylesterase polymorphism in a Turkish population. Pharmacogenetics 1:58–61
Serdar Z, Aslan K, Dirican M, Sarandol E, Yesilbursa D, Serdar A (2006) Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem 39:794–803
Elkiran ET, Mar N, Aygen B, Gursu F, Karaoglu A, Koca S (2007) Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 7:48–55
Gan KN, Smolen A, Eckerson HW, La Du BN (1991) Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 19:100–106
Kataria RK, Brent LH (2004) Spondyloarthropathies. Am Fam Physician 69:2853–2860
Karakoc M, Altindag O, Keles H, Soran N, Selek S (2007) Serum oxidative–antioxidative status in patients with ankylosing spondilitis. Rheumatol Int 27:1131–1134
Erdem FH, Karatay S, Yildirim K, Kiziltunc A (2010) Evaluation of serum paraoxonase and arylesterase activities in ankylosing spondylitis patients. Clinics 65(2):175–179
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–2285
Eckerson HW, Wyte MC, La Du BN (1983) The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 35:1126–1138
Haagen L, Brock A (1992) A new automated method for phenotyping arylesterase (E.C.3.1.1.2.) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clim Biochem 30:391–395
Ohkawa H, Ohisi N, Yagi K (1979) Assay for peroxides in animal tissue by thiobarbituric acid reaction. Ann Biochem 95:351–358
Reddy ST, Wadleigh DJ, Grijalva V et al (2001) Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 21:542–547
Collins AR (2005) Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. Am J Clin Nutr 81(suppl 1):261S–267S
Tanimoto N, Kumon Y, Suehiro T, Ohkubo S et al (2003) Serum paraoxonase activity decreases in rheumatoid arthritis. Life Sci 72:2877–2885
Baskol G, Demir H, Baskol M, Kilic E et al (2005) Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem 38:951–955
Ozgocmen S, Sogut S, Fadıllıoglu E, Ardicoglu A, Ardicoglu O (2003) Antioxidant status and lipid peroxidation in seminal plasma and spermatozoa of patients with ankylosing spondylitis. Rheumatology 42:805–807
Ozgocmen S, Sogut S, Ardicoglu O, Fadillioglu E, Pekkutucu I, Akyol O (2004) Serum nitric oxide, catalase, superoxide dismutase, and malondialdehyde status in patients with ankylosing spondylitis. Rheumatol Int 24:80–83
Stanek A, Cieślar G, Romuk E, Kasperczyk S, Sieroń-Stołtny K, Birkner E, Sieroń A (2010) Decrease in antioxidant status of plasma and erythrocytes from patients with ankylosing spondylitis. Clin Biochem 43(6):566–570 (epub 4 Jan 2010)
Cece H, Yazgan P, Karakas E, Karakas O, Demirkol A, Toru I, Aksoy N (2011) Carotid intima-media thickness and paraoxonase activity in patients with ankylosing spondylitis. Clin Invest Med 34(4):E225
Canales A, Sanchez-Muniz FJ (2003) Paraoxanase, something more than an enzyme? Med Clin (Barc) 121:537–548 (Spanish)
Steinberg D (1997) Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 272:20963–20966
Laplaud PM, Dantoine T, Chapman MJ (1998) Paraoxonase as a risk marker for cardiovascular disease: facts and hypotheses. Clin Chem Lab Med 37:431–441
Jakubowski H (2005) Anti-N-homocysteinylated protein autoantibodies and cardiovascular disease. Clin Chem Lab Med 43:1011–1014
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olama, S.M., Elarman, M.M. Evaluation of paraoxonase and arylesterase activities in Egyptian patients with ankylosing spondylitis. Rheumatol Int 33, 1487–1494 (2013). https://doi.org/10.1007/s00296-012-2591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2591-1