Abstract
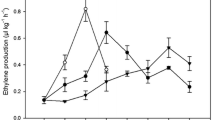
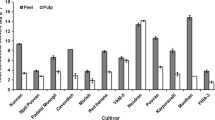
β-1,3 glucanase (E.C.3.2.1.39) is the key enzyme involved in the hydrolytic cleavage of 1,3 β-D glucosidic linkages in β-1,3 glucans. This work describes a comparative analysis of expression patterns of β-1,3 glucanase gene in relation to changes in fruit pulp softening rates in three banana cultivars, Rasthali (AAB), Kanthali (AB), and Monthan (ABB). Analysis of transcript and protein levels of β-1,3 glucanase gene during ripening revealed differential timing in expression of the gene which correlated well with the variation in enzymatic activity of glucanase and fruit pulp softening rates in the three cultivars. Exogenously applied ethylene strongly induced β-1,3 glucanase expression during the early ripening days in Rasthali, while the expression of the gene was marginally stimulated following ethylene treatment in preclimacteric Kanthali fruit. Conversely, in Monthan, β-1,3 glucanase expression was very low throughout the ripening stages, and ethylene treatment did not induce the expression of the gene in this cultivar. Analysis of glucanase activity using protein extracts from unripe and ripe fruit of Monthan with crude cell wall polysaccharide fractions (used as substrate) indicated that the natural substrate for glucanase remained almost unutilized in this cultivar due to low in vivo glucanase activity. Furthermore, the recombinant β-1,3 glucanase protein, overexpressed in E. coli, showed requirement for substrates with contiguous β-1,3 linkages for optimal activity. Overall, our results provide new information on the expression profile of β-1,3 glucanase gene in connection with the pattern of changes in fruit firmness at the physiological and molecular levels during ripening in three banana cultivars.





Similar content being viewed by others
Change history
11 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00299-023-03122-6
References
Abeles FB, Bosshart RP, Forrence LE, Habig WH (1970) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47:129–134
Ali ZM, Ng SY, Othman R, Goh LY, Lazan H (1998) Isolation, characterization and significance of papaya β-galactanases to cell wall modifications and fruit softening during ripening. Physiol Plant 104:105–115
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Brady CJ (1987) Fruit ripening. Annu Rev Plant Physiol 38:155–173
Brummell DA, Cin VD, Crisosto CH, Labavitch JM (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot 55:2029–2039
Bustamante CA, Rosli HG, Anon MC, Civello PM, Martinez GA (2006) β-Xylosidase in strawberry fruit: isolation of a full-length gene and analysis of its expression and enzymatic activity in cultivars with contrasting firmness. Plant Sci 171:497–504
Chin LH, Ali ZM, Lazan H (1999) Cell wall modifications, degrading enzymes and softening of Carambola fruit during ripening. J Exp Bot 50:767–775
Clendennen KS, May DG (1997) Differential gene expression in ripening banana fruit. Plant Physiol 115:463–469
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Physiol Plant 125:131–134
Dong J-Z, Dunstan DI (1997) Endochitinase and β-1,3-glucanase genes are developmentally regulated during somatic embryogenesis in Picea glauca. Planta 201:189–204
Fischer RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Plant Mol Biol 42:675–703
Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL (1992) Polygalacturonase and tomato fruit ripening. Hortic Rev 13:67–109
Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB (1998) Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening associated pectin disassembly. Physiol Plant 117:363–373
Hall CB (1987) Firmness of tomato tissues according to cultivars and ripeness. J Am Soc Hortic Sci 112:663–665
Hird DL, Worrall D, Hodge R, Smartt S, Paul W, Scott R (1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6 gene displays similarity to β-1,3-glucanases. Plant J 4:1023–1033
Kesari R, Trivedi PK, Nath P (2007) Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Biol Technol 46:136–143
Kojima K, Sakurai N, Kuraishi S (1994) Fruit softening in banana: correlation among stress-relaxation parameters, cell wall components and starch during ripening. Physiol Plant 90:772–778
Krabel D, Eschrich W, Wirth S, Wolf G (1993) Callase-(1,3-β-D-glucanase) activity during spring reactivation in deciduous trees. Plant Sci 93:19–23
Leubner-Metzger G, Meins F Jr (1999) Functions and regulation of plant β-1,3-glucanases (PR-2), Review. In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants. CRC Press LLC, Boca Raton, pp 49–76
McCollum TG, Huber DJ, Cantliffe DJ (1989) Modification of polyuronides and hemicelluloses during muskmelon fruit softening. Physiol Plant 76:303–308
Micheli F (2001) Pectin methylesterase: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6:414–419
Mitcham EJ, McDonald RE (1992) Cell wall modification during ripening of ‘Keitt’ and ‘Tommy Atkins’ mango fruit. J Am Soc Hortic Sci 117:919–924
Muda P, Seymour GB, Errington N, Tucker GA (1995) Compositional changes in cell wall polymers during mango fruit ripening. Carbohydr Polym 26:255–260
Ori N, Sessa G, Lotan T, Himmelhoch S, Fluhr R (1990) A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related proteins superclass. EMBO J 9:3429–3436
Peumans WJ, Barre A, Derycke V, Rougé P, Zhang W, May GD, Delcour JA, Leuven FV, Van Damme EJM (2000) Purification, characterization and structural analysis of an abundant -1,3-glucanase from banana fruit. Eur J Biochem 267:1188–1195
Redgwell RJ, Melton LD, Brasch DJ (1992) Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa). Solubilization of the pectic polymers. Physiol Plant 98:71–81
Rose JKC, Bennett AB (1999) Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci 4:176–183
Roy Choudhury S, Roy S, Saha PP, Singh SK, Sengupta DN (2008a) Characterization of differential ripening pattern in association with ethylene biosynthesis in the fruits of five naturally occurring banana cultivars and detection of a GCC-box specific DNA binding protein. Plant Cell Rep 27:1235–1249
Roy Choudhury S, Roy S, Sengupta DN (2008b) Characterization of transcriptional profiles of MA-ACS1 and MA-ACO1 genes in response to ethylene, auxin, wounding, cold and different photoperiods during ripening in banana fruit. J Plant Physiol 165:1865–1878
Scott KJ, McGlasson WB, Roberts EA (1970) Potassium permanganate as an ethylene absorbent in polyethylene bags to delay ripening of bananas during storage. Aust J Exp Agric 10:237–240
Shi Y, Zhang Y, Shih DS (2006) Cloning and expression analysis of two β-1,3-glucanase genes from Strawberry. J Plant Physiol 163:956–967
Smith DL, Gross KC (2000) A family of at least seven β-galactosidase genes is expressed during tomato fruit development. Plant Physiol 123:1173–1183
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24:1596–1599
Trivedi P, Nath P (2004) MaExp1, an ethylene-induced expansion from ripening banana fruit. Plant Sci 6:1351–1358
Von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14:4683–4690
Acknowledgments
We thank Prof. Frederick Meins, FMIBR, Basel, Switzerland for providing the antibody of β-1,3 glucanase. We gratefully acknowledge the financial assistance from Council for Scientific and Industrial Research (CSIR). We thank Mr. Jadav Ghosh, Department of Botany, Bose Institute, for the necessary technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
S. Roy Choudhury and S. Roy contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2009_764_MOESM1_ESM.tif
Supplementary Fig 1. The phylogenetic tree was constructed based on the deduced amino acid sequences of different fruit-specific β 1,3 glucanases. The tree was constructed using neighbor joining method with the best tree mode by Mega4.1 version. A value of 0.1 corresponds to a difference of 10% between the two sequences (TIFF 723 kb)
Rights and permissions
About this article
Cite this article
Roy Choudhury, S., Roy, S. & Sengupta, D.N. Characterization of cultivar differences in β-1,3 glucanase gene expression, glucanase activity and fruit pulp softening rates during fruit ripening in three naturally occurring banana cultivars. Plant Cell Rep 28, 1641–1653 (2009). https://doi.org/10.1007/s00299-009-0764-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0764-5