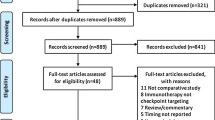
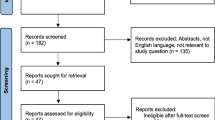
Abstract
Objectives
To compare the incidence of treatment-related necrosis between combination SRS+ICI therapy and SRS therapy alone in patients with brain metastases from melanoma and non-small cell lung cancer (NSCLC).
Methods
A systematic literature search of Ovid-MEDLINE and EMBASE was performed up to August 10, 2020. The difference in the pooled incidence of treatment-related necrosis after SRS+ICI or SRS alone was evaluated. The cumulative incidence of treatment-related necrosis at the specific time point after the treatment was calculated and plotted. Subgroup and meta-regression analyses were additionally performed.
Results
Sixteen studies (14 on melanoma, 2 on NSCLC) were included. In NSCLC brain metastasis, the reported incidences of treatment-related necrosis in SRS+ICI and SRS alone ranged 2.9–3.4% and 0–2.9%, respectively. Meta-analysis was conducted including 14 studies on melanoma brain metastasis. The incidence of treatment-related necrosis was higher in SRS+ICI than SRS alone (16.0% vs. 6.5%; p = 0.065; OR, 2.35). The incidence showed rapid increase until 12 months after the SRS when combined with ICI therapy (14%; 95% CI, 8–22%) and its pace of increase slowed thereafter. Histopathologic diagnosis as the reference standard for treatment-related necrosis and inclusion of only symptomatic cases were the source of heterogeneity in SRS+ICI.
Conclusions
Treatment-related necrosis tended to occur 2.4 times more frequently in the setting of combination SRS+ICI therapy compared with SRS alone in melanoma brain metastasis showing high cumulative incidence within the first year. Treatment-related necrosis should be considered when SRS+ICI combination therapy is used for melanoma brain metastasis, especially in the first year.
Key Points
• Treatment-related necrosis occurred 2.4 times more frequently in the setting of combination SRS+ICI therapy compared with SRS alone in melanoma brain metastasis.
• Treatment-related necrosis more frequently occurred in brain metastases from melanoma than NSCLC.
• Reference standard for treatment-related necrosis and inclusion of only symptomatic treatment-related necrosis were a significant source of heterogeneity, indicating varying definitions of treatment-related necrosis in the literature need to be unified.




Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- ICI:
-
Immune checkpoint inhibitor
- KM curve:
-
Kaplan-Meier curve
- NSCLC:
-
Non-small cell lung cancer
- SRS:
-
Stereotactic radiosurgery
References
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872
Schouten LJ, Rutten J, Huveneers HA, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–2705
Cagney DN, Martin AM, Catalano PJ et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19:1511–1521
Sampson JH, Carter JH Jr, Friedman AH, Seigler HF (1998) Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 88:11–20
Amer MH, Al-Sarraf M, Vaitkevicius VK (1979) Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet 149:687–692
Budman DR, Camacho E, Wittes RE (1978) The current causes of death in patients with malignant melanoma. Eur J Cancer 14:327–330
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9:180–188
Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 32:1885–1892
Shepard MJ, Xu Z, Donahue J et al (2019) Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. https://doi.org/10.3171/2019.4.Jns19822:1-8
Minniti G, Anzellini D, Reverberi C et al (2019) Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer 7:102
Diao K, Bian SX, Routman DM et al (2018) Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg 129:1397–1406
Martin AM, Cagney DN, Catalano PJ et al (2018) Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol 4:1123–1124
Kaidar-Person O, Zagar TM, Deal A et al (2017) The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs 28:669–675
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Atkins D, Eccles M, Flottorp S et al (2004) Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 4:38
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Kim KW, Lee J, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol 16:1175–1187
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–1196
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Sterne JA, Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 323:101–105
Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906
Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL (2012) Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 117:227–233
Skrepnik T, Sundararajan S, Cui H, Stea B (2017) Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 6:e1283461
Pires da Silva I, Glitza IC, Haydu LE et al (2019) Incidence, features and management of radionecrosis in melanoma patients treated with cerebral radiotherapy and anti-PD-1 antibodies. Pigment Cell Melanoma Res 32:553–563
Patel KR, Shoukat S, Oliver DE et al (2017) Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 40:444–450
Nardin C, Mateus C, Texier M et al (2018) Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res 28:111–119
Kotecha R, Miller JA, Venur VA et al (2018) Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg 129:50–59
Du Four S, Janssen Y, Michotte A et al (2018) Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med 7:4870–4879
Robin TP, Breeze RE, Smith DE et al (2018) Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. J Neurooncol 140:55–62
Olson AC, Thomas S, Qin R et al (2016) Outcomes and toxicity of stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab. Melanoma Manag 3:177–186
Hubbeling HG, Schapira EF, Horick NK et al (2018) Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non–small cell lung cancer. J Thorac Oncol 13:550–558
Fang P, Jiang W, Allen P et al (2017) Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol 133:595–602
Yang L, Liu L, Wu X, Guo X, Yang Y, Wang T (2020) Hypofractionated radiation therapy with versus without immune checkpoint inhibitors in patients with brain metastases: a meta-analysis. Int Immunopharmacol 80:106148
Anderson ES, Postow MA, Wolchok JD et al (2017) Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 5:76
Trommer-Nestler M, Marnitz S, Kocher M et al (2018) Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci 19:2653
Galldiks N, Kocher M, Ceccon G et al (2020) Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol 22:17–30
Alomari AK, Cohen J, Vortmeyer AO et al (2016) Possible interaction of Anti-PD-1 therapy with the effects of radiosurgery on brain metastases. Cancer Immunol Res 4:481–487
Rauch PJ, Park HS, Knisely JP, Chiang VL, Vortmeyer AO (2012) Delayed radiation-induced vasculitic leukoencephalopathy. Int J Radiat Oncol Biol Phys 83:369–375
Alomari A, Rauch PJ, Orsaria M, Minja FJ, Chiang VL, Vortmeyer AO (2014) Radiologic and histologic consequences of radiosurgery for brain tumors. J Neurooncol 117:33–42
Winter SF, Loebel F, Loeffler J et al (2019) Treatment-induced brain tissue necrosis: a clinical challenge in neuro-oncology. Neuro Oncol 21:1118–1130
Alexander BM, Brown PD, Ahluwalia MS et al (2018) Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 19:e33–e42
Camidge DR, Lee EQ, Lin NU et al (2018) Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol 19:e20–e32
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542
Na A, Haghigi N, Drummond KJ (2014) Cerebral radiation necrosis. Asia Pac J Clin Oncol 10:11–21
van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27:4129–4144
Kim SJ, Ryul Shim S (2019) Diagnostic value of radiolabeled amino acid PET for detection of pseudoprogression of brain tumor after treatment: a meta-analysis. Nucl Med Commun 40:965–972
Nishino M, Hatabu H, Hodi FS (2019) Imaging of cancer immunotherapy: current approaches and future directions. Radiology 290:9–22
Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A (2018) Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol 28:401–411
Park HJ, Kim KW, Pyo J et al (2020) Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 297:87–96
Goyal S, Silk AW, Tian S et al (2015) Clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol 1:668–676
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chong Hyun Suh.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this study is a systematic review and meta-analysis.
Ethical approval
Ethical approval was not required for this study because this study is a systematic review and meta-analysis.
Methodology
• Meta-analysis
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, P.H., Suh, C.H., Kim, H.S. et al. Immune checkpoint inhibitor therapy may increase the incidence of treatment-related necrosis after stereotactic radiosurgery for brain metastases: a systematic review and meta-analysis. Eur Radiol 31, 4114–4129 (2021). https://doi.org/10.1007/s00330-020-07514-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07514-0