Abstract
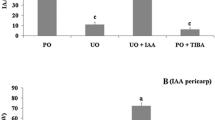
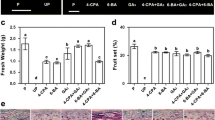
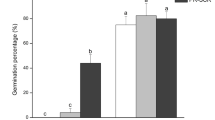
In this work, we study the capacity to biosynthesize gibberellins (GA) of ovules (either fertilised or unfertilised), developing seeds and pericarp from fruitlets and their relation with fruit set capacity. Experiments were performed in adult, 12-year-old trees of seeded (Pineapple) and seedless parthenocarpic (Washington navel) sweet orange [Citrus sinensis L. Osbeck] cultivars. The activity of GA20-, GA3- and GA2-oxidases and gibberellin levels were measured in the ovules and pericarp of fruitlets in different development states. The results indicate that ovules are the main sites of gibberellin synthesis in fruitlets during the post-anthesis period. The most intense GA1 synthesis—coincident with the highest expression of GA20ox2, GA3ox1 and GA2ox1—was detected in the ovules of the seeded cultivar, probably induced by fecundation and associated with low early fruitlet abscission rates. By contrast, the low activity detected in the sterile cultivar appears to be rather developmentally or constitutively regulated. As a fruitlet develops, the GA1 concentration is augmented in the pericarp in comparison to ovules or developing seeds, and levels therein did not exhibit noticeable differences between varieties. Furthermore, developing seeds from pineapple had higher GA1 content than the unfertilised abortive ovules from Washington navel. Taken together, data suggest a main role for this hormone in the control of fruitlet abscission, and also demonstrate a function in seed development.






Similar content being viewed by others
References
Ben-Cheikh W, Perez-Botella J, Tadeo FR, Talón M, Primo-Millo E (1997) Pollination increases gibberellin levels in developing ovaries of seeded varieties of citrus. Plant Physiol 114:557–564
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39
De Jong M, Mariani C, Vriezen WH (2009) The role of auxin and gibberellin in tomato fruit set. J Exp Bot 60:1523–1532
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Pérez-Amador MA (2009) Fertilization-depend auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Eeuwens CJ, Schwabe WW (1975) Seed and pod wall development in Pisum sativum L. in relation to extracted and applied hormones. J Exp Bot 26:1–14
Erickson L, Brannaman BL (1960) Abscission of reproductive structures and leaves of orange trees. J Am Soc Hort Sci 75:222–229
Fos M, Nuez F, García-Martinez JL (2000) The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–480
Fos M, Proano K, Nuez F, García-Martinez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111:545–550
Frost HB, Soost RK (1968) Seed reproduction: development of gametes and embryos. In: Reuther W, Batchelor LD, Webber HJ (eds) The Citrus Industry, vol 2., University of CaliforniaCalifornia, USA, pp 290–320
García-Hurtado N, Carrera E, Ruíz-Rivero O, López-Gresa MP, Hedden P, Gong F, García-Martínez JL (2012) The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J Exp Bot 63:5803–5813
García-Martinez JL, García-Papi MA (1979) The influence of gibberellic acid, 2,4-dichlorophenoxyacetic acid and 6-benzylaminopurine on fruit set of Clementine mandarin. Sci Hort 10:285–293
García-Martínez JL, Martí M, Sabater T, Maldonado A, Vercher Y (1991a) Development of fertilized ovules and their role in the growth of pea pod. Physiol Plant 83:411–416
García-Martínez JL, Santes C, Croker SJ, Hedden P (1991b) Identification, quantitation, and distribution of gibberellins in fruits of Pisum sativum cv. Alaska during pod development. Planta 184:53–60
García-Martínez JL, López-Díaz M, Sanchez-Beltrán MJ, García-Martínez JL, Phillips AL, Ward DA, Gaskin P, Hedden P (1997) Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol 33:1073–1084
Giacomelli L, Rota-Stabelli O, Masuero D, Acheampong AK, Moretto M, Caputi L (2013) Gibberellin metabolism in Vitis vinifera L. during bloom and fruit set: functional characterization and evolution of grapevine gibberellin oxidases. J Exp Bot 64:4403–4419
Gómez-Cadenas A, Mehouachi J, Tadeo FR, Primo-Millo E, Talón M (2000) Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 210:636–643
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acid Res 40:D1178–D1186 (Database issue)
Guardiola JL, García-Marí F, Agustí M (1984) Competition and fruit set in the Washington Navel orange. Physiol Plant 62:297–302
Hazra P, Dutta AK, Chatterjee P (2010) Altered gibberellin and auxin levels in the ovaries in the manifestation of genetic parthenocarpy in tomato (Solanum lycopersicum). Curr Sci 99:1439–1443
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25
Huerta L, García-Lor A, García-Martínez JL (2009) Characterization of gibberellin 20-oxidases in the citrus hybrid Carrizo citrange. Tree Physiol 29:569–577
Iglesias DJ, Tadeo FR, Primo-Millo E, Talón M (2006) Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 20:348–355
Mariotti L, Picciarelli P, Lombardi L, Ceccarelli N (2011) Fruit set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J Plant Growth Reg 30:405–415
Martí E, Carrera E, Ruiz-Rivero O, García-Martínez JL (2010) Hormonal regulation of tomato gibberellin 20-oxidase1 expressed in Arabidopsis. J Plant Physiol 167:1188–1196
Medina M, Roque E, Oineda B, Cañas L, Rodriguez-Concepción M, Beltrán JP, Gómez-Mena C (2013) Early anther ablation triggers parthenocarpic fruit development in tomato. Plant Biotechnol J 11:770–779
Mehouachi J, Serna D, Zaragoza S, Agustí M, Talón M, Primo Millo E (1995) Defoliation increases fruit abscission and reduces carbohydrate levels in developing fruits and woody tissues of Citrus unshiu. Plant Sci 107:189–197
Mehouachi J, Iglesias DJ, Tadeo FR, Agustí M, Primo-Millo E, Talón M (2000) The role of leaves in citrus fruitlet abscission: effects on endogenous gibberellins levels and carbohydrate content. J Hort Sci Biotechnol 75:79–85
Mesejo C, Yuste R, Martinez-Fuentes A, Reig C, Iglesias DJ, Primo-Millo E, Agustí M (2013) Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (C. clementina). Physiol Plant 148:187–196
O´Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue 0. Protoplasma 59:368–373
Ollimpieri I, Siligato F, Caccia R, Mariotti L, Ceccarelli N, Soressi GP, Mazzucato A (2007) Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 226:877–888
Ozga JA, Reinecke DM (2003) Hormonal interactions in fruit development. J Plant Growth Reg 22:73–81
Ozga JA, Reinecke DM, Ayele BT, Ngo P, Nadeau C, Wickramarathna AD (2009) Developmental and hormonal regulation of gibberellin biosynthesis and catabolism in pea fruit. Plant Physiol 150:448–462
Poling SM (1991) Identification of endogenous gibberellins in immature navel orange fruit. J Agric Food Chem 39:677–680
Rodrigo MJ, García-Martínez JL, Santes CM, Gaskin P, Hedden P (1997) The role of gibberellins A(1) and A(3) in fruit growth of Pisum sativum L and the identification of gibberellins A(4) and A(7) in young seeds. Planta 201:446–455
Ruan YL, Patric JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17:656–665
Santes CM, Hedden P, Gaskin P, García-Martínez JL (1995) Gibberellins and related compounds in young fruits of pea and their relationship to fruit set. Phytochemistry 40:1347–1355
Seo M, Jikumaru Y, Kamiya Y (2011) Profiling of hormones and related metabolites in seed dormancy and germination studies. Methods Mol Biol 773:99–111
Serfontein CM, Catling HD (1968) Determining the canopy area of citrus trees. S Afr Citrus J 413:14–15
Serrani JC, Fos M, Atares A, García-Martínez JL (2007a) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv micro-torn of tomato. J Plant Growth Reg 26:211–221
Serrani JC, Sanjuan R, Ruiz-Rivero O, Fos M, García-Martínez JL (2007b) Gibberellin regulation of fruit set and growth in tomato. Plant Physiol 145:246–257
Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Tadeo FR, Tudela D, Primo-Millo E (1995) 1-Aminocyclopropane-1- carboxylic acid-induced ethylene stimulates callus formation by cell enlargement in the cambial region of internodal explants of citrus. Plant Sci 110:113–119
Talón M, Hedden P, Primo-Millo E (1990a) Gibberellins in Citrus sinensis: a comparison between seeded and seedless varieties. J Plant Growth Reg 9:201–206
Talón M, Zacarías L, Primo-Millo E (1990b) Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol Plant 79:400–406
Talón M, Zacarías L, Primo-Millo E (1992) Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol 99:1575–1581
Turnbull GCN (1989) Identification and quantitative analysis of gibberellins in Citrus. J Plant Growth Reg 8:273–282
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Ann Rev Plant Biol 59:225–251
Yan J, Yuan F, Long G, Qin L, Deng Z (2012) Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol Biol Reports 39:1831–1838
Acknowledgments
We thank Drs. Isabel Lopez-Diaz and Esther Carrera for the hormone quantification carried out at the Plant Hormone Quantification Service, IBMCP, Valencia, Spain. Thanks are due to Teresa Sabater from the IBMCP, for their help. This work has been supported by two research projects, RTA2013-00024-CO2-01 from INIA (Ministerio de Economía y Competitividad, Spain) and IVIA-5423 from Consellería de Agricultura (Generalitat Valenciana, Valencia, Spain).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bermejo, A., Martínez-Alcántara, B., Martínez-Cuenca, MR. et al. Biosynthesis and Contents of Gibberellins in Seeded and Seedless Sweet Orange (Citrus sinensis L. Osbeck) Cultivars. J Plant Growth Regul 35, 1036–1048 (2016). https://doi.org/10.1007/s00344-016-9602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9602-5