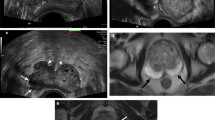
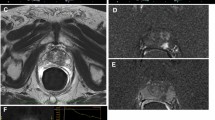
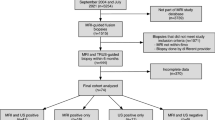
Abstract
The diagnosis of prostate cancer (PCa) can be challenging due to the limited performance of current diagnostic tests, including PSA, digital rectal examination and transrectal conventional US. Multiparametric MRI has improved PCa diagnosis and is recommended prior to biopsy; however, mp-MRI does miss a substantial number of PCa. Advanced US modalities include transrectal prostate elastography and contrast-enhanced US, as well as improved B-mode, micro-US and micro-Doppler techniques. These techniques can be combined to define a novel US approach, multiparametric US (mp-US). Mp-US improves PCa diagnosis but is not sufficiently accurate to obviate the utility of mp-MRI. Mp-US using advanced techniques and mp-MRI provide complementary information which will become even more important in the era of focal therapy, where precise identification of PCa location is needed.












Similar content being viewed by others
References
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629. https://doi.org/10.1016/j.eururo.2016.08.003
Hayes JH, Barry MJ (2014) Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 311:1143–1149. https://doi.org/10.1001/jama.2014.2085
Olleik G, Kassouf W, Aprikian A et al (2018) Evaluation of new tests and interventions for prostate cancer management: a systematic review. J Natl Compr Cancer Netw JNCCN 16:1340–1351. https://doi.org/10.6004/jnccn.2018.7055
Kelloff GJ, Choyke P, Coffey DS, Prostate Cancer Imaging Working Group (2009) Challenges in clinical prostate cancer: role of imaging. AJR Am J Roentgenol 192:1455–1470. https://doi.org/10.2214/AJR.09.2579
Mian BM, Naya Y, Okihara K et al (2002) Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology 60:836–840. https://doi.org/10.1016/s0090-4295(02)01950-7
Singh H, Canto EI, Shariat SF et al (2004) Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol 171:1850–1854. https://doi.org/10.1097/01.ju.0000119667.86071.e7
Delongchamps NB, Haas GP (2009) Saturation biopsies for prostate cancer: current uses and future prospects. Nat Rev Urol 6:645–652. https://doi.org/10.1038/nrurol.2009.213
Giannarini G, Autorino R, di Lorenzo G (2009) Saturation biopsy of the prostate: why saturation does not saturate. Eur Urol 56:619–621. https://doi.org/10.1016/j.eururo.2009.03.044
Ashley RA, Inman BA, Routh JC et al (2008) Reassessing the diagnostic yield of saturation biopsy of the prostate. Eur Urol 53:976–981. https://doi.org/10.1016/j.eururo.2007.10.049
Nougaret S, Robertson N, Golia Pernicka J et al (2017) The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol NY 42:1968–1974. https://doi.org/10.1007/s00261-017-1086-7
Bloom JB, Hale GR, Gold SA et al (2019) Predicting Gleason Group progression for men on prostate cancer active surveillance: role of a negative confirmatory magnetic resonance imaging-ultrasound fusion biopsy. J Urol 201:84–90. https://doi.org/10.1016/j.juro.2018.07.051
Richenberg J, Løgager V, Panebianco V et al (2019) The primacy of multiparametric MRI in men with suspected prostate cancer. Eur Radiol 29:6940–6952. https://doi.org/10.1007/s00330-019-06166-z
Faria R, Soares MO, Spackman E et al (2018) Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol 73:23–30. https://doi.org/10.1016/j.eururo.2017.08.018
Rouvière O, Puech P, Renard-Penna R et al (2019) Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 20:100–109. https://doi.org/10.1016/S1470-2045(18)30569-2
Vargas HA, Akin O, Shukla-Dave A et al (2012) Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 265:478–487. https://doi.org/10.1148/radiol.12120041
Fütterer JJ, Briganti A, De Visschere P et al (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68:1045–1053. https://doi.org/10.1016/j.eururo.2015.01.013
Johnson DC, Raman SS, Mirak SA et al (2019) Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 75:712–720. https://doi.org/10.1016/j.eururo.2018.11.031
Niaf É, Flamary R, Rouvière O et al (2014) Kernel-based learning from both qualitative and quantitative labels: application to prostate cancer diagnosis based on multiparametric MR imaging. IEEE Trans Image Process Publ IEEE Signal Process Soc 23:979–991. https://doi.org/10.1109/TIP.2013.2295759
Dinh AH, Melodelima C, Souchon R et al (2018) Characterization of prostate cancer with gleason score of at least 7 by using quantitative multiparametric mr imaging: validation of a computer-aided diagnosis system in patients referred for prostate biopsy. Radiology 287:525–533. https://doi.org/10.1148/radiol.2017171265
Norberg M, Egevad L, Holmberg L et al (1997) The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology 50:562–566. https://doi.org/10.1016/S0090-4295(97)00306-3
Beerlage HP, Aarnink RG, Ruijter ET et al (2001) Correlation of transrectal ultrasound, computer analysis of transrectal ultrasound and histopathology of radical prostatectomy specimen. Prostate Cancer Prostatic Dis 4:56–62. https://doi.org/10.1038/sj.pcan.4500495
Cheng S, Rifkin MD (2001) Color Doppler imaging of the prostate: important adjunct to endorectal ultrasound of the prostate in the diagnosis of prostate cancer. Ultrasound Q 17:185–189. https://doi.org/10.1097/00013644-200109000-00008
Zhai L, Madden J, Foo W-C et al (2010) Characterizing stiffness of human prostates using acoustic radiation force. Ultrason Imaging 32:201–213. https://doi.org/10.1177/016173461003200401
Baumgart LA, Gerling GJ, Bass EJ (2010) Characterizing the range of simulated prostate abnormalities palpable by digital rectal examination. Cancer Epidemiol 34:79–84. https://doi.org/10.1016/j.canep.2009.12.002
Smith DS, Catalona WJ (1995) Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 45:70–74. https://doi.org/10.1016/s0090-4295(95)96812-1
Lughezzani G, Saita A, Lazzeri M et al (2019) Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. Eur Urol Oncol 2:329–332. https://doi.org/10.1016/j.euo.2018.10.001
Loch T (2007) Computerized transrectal ultrasound (C-TRUS) of the prostate: detection of cancer in patients with multiple negative systematic random biopsies. World J Urol 25:375–380. https://doi.org/10.1007/s00345-007-0181-8
Strunk T, Decker G, Willinek W et al (2014) Combination of C-TRUS with multiparametric MRI: potential for improving detection of prostate cancer. World J Urol 32:335–339. https://doi.org/10.1007/s00345-012-0924-z
Braeckman J, Autier P, Soviany C et al (2008) The accuracy of transrectal ultrasonography supplemented with computer-aided ultrasonography for detecting small prostate cancers. BJU Int 102:1560–1565. https://doi.org/10.1111/j.1464-410X.2008.07878.x
Sivaraman A, Sanchez-Salas R, Barret E et al (2015) Prostate histoscanning true targeting guided prostate biopsy: initial clinical experience. World J Urol 33:1475–1479. https://doi.org/10.1007/s00345-014-1434-y
Schiffmann J, Mehring G, Tennstedt P et al (2016) True targeting-derived prostate biopsy: HistoScanningTM remained inadequate despite advanced technical efforts. World J Urol 34:495–500. https://doi.org/10.1007/s00345-015-1637-x
Wysock JS, Xu A, Orczyk C, Taneja SS (2017) Histo scanning TM to detect and characterize prostate cancer-a review of existing literature. Curr Urol Rep 18:97. https://doi.org/10.1007/s11934-017-0747-y
Wildeboer RR, van Sloun RJG, Wijkstra H, Mischi M (2020) Artificial intelligence in multiparametric prostate cancer imaging with focus on deep-learning methods. Comput Methods Progr Biomed 189:105316. https://doi.org/10.1016/j.cmpb.2020.105316
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659. https://doi.org/10.1056/NEJM198612253152606
Tuxhorn JA, Ayala GE, Rowley DR (2001) Reactive stroma in prostate cancer progression. J Urol 166:2472–2483
Barron DA, Rowley DR (2012) The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer 19:R187–204. https://doi.org/10.1530/ERC-12-0085
Tuxhorn JA, Ayala GE, Smith MJ et al (2002) Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8:2912–2923
Phipps S, Yang THJ, Habib FK et al (2005) Measurement of tissue mechanical characteristics to distinguish between benign and malignant prostatic disease. Urology 66:447–450. https://doi.org/10.1016/j.urology.2005.03.017
Hoyt K, Castaneda B, Zhang M et al (2008) Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark Sect Dis Markers 4:213–225. https://doi.org/10.3233/cbm-2008-44-505
Zhang M, Nigwekar P, Castaneda B et al (2008) Quantitative characterization of viscoelastic properties of human prostate correlated with histology. Ultrasound Med Biol 34:1033–1042. https://doi.org/10.1016/j.ultrasmedbio.2007.11.024
Ahn B-M, Kim J, Ian L et al (2010) Mechanical property characterization of prostate cancer using a minimally motorized indenter in an ex vivo indentation experiment. Urology 76:1007–1011. https://doi.org/10.1016/j.urology.2010.02.025
Carson WC, Gerling GJ, Krupski TL et al (2011) Material characterization of ex vivo prostate tissue via spherical indentation in the clinic. Med Eng Phys 33:302–309. https://doi.org/10.1016/j.medengphy.2010.10.013
Barr RG, Cosgrove D, Brock M et al (2017) WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 5. Prostate Ultrasound Med Biol 43:27–48. https://doi.org/10.1016/j.ultrasmedbio.2016.06.020
Tsutsumi M, Miyagawa T, Matsumura T et al (2010) Real-time balloon inflation elastography for prostate cancer detection and initial evaluation of clinicopathologic analysis. AJR Am J Roentgenol 194:W471–476. https://doi.org/10.2214/AJR.09.3301
Miyagawa T, Tsutsumi M, Matsumura T et al (2009) Real-time elastography for the diagnosis of prostate cancer: evaluation of elastographic moving images. Jpn J Clin Oncol 39:394–398. https://doi.org/10.1093/jjco/hyp026
Junker D, Schäfer G, Aigner F et al (2012) Potentials and limitations of real-time elastography for prostate cancer detection: a whole-mount step section analysis. Sci World J 2012:193213. https://doi.org/10.1100/2012/193213
Junker D, Schäfer G, Kobel C et al (2014) Comparison of real-time elastography and multiparametric MRI for prostate cancer detection: a whole-mount step-section analysis. AJR Am J Roentgenol 202:W263–269. https://doi.org/10.2214/AJR.13.11061
Sumura M, Shigeno K, Hyuga T et al (2007) Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: a preliminary study. Int J Urol Off J Jpn Urol Assoc 14:811–816. https://doi.org/10.1111/j.1442-2042.2007.01829.x
Zhu Y, Chen Y, Qi T et al (2014) Prostate cancer detection with real-time elastography using a bi-plane transducer: comparison with step section radical prostatectomy pathology. World J Urol 32:329–333. https://doi.org/10.1007/s00345-012-0922-1
Zhang B, Ma X, Zhan W et al (2014) Real-time elastography in the diagnosis of patients suspected of having prostate cancer: a meta-analysis. Ultrasound Med Biol 40:1400–1407. https://doi.org/10.1016/j.ultrasmedbio.2014.02.020
Pallwein L, Mitterberger M, Struve P et al (2007) Comparison of sonoelastography guided biopsy with systematic biopsy: impact on prostate cancer detection. Eur Radiol 17:2278–2285. https://doi.org/10.1007/s00330-007-0606-1
Aigner F, Pallwein L, Junker D et al (2010) Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less. J Urol 184:913–917. https://doi.org/10.1016/j.juro.2010.05.026
Brock M, von Bodman C, Palisaar RJ et al (2012) The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: a prospective study of 353 patients. J Urol 187:2039–2043. https://doi.org/10.1016/j.juro.2012.01.063
Wang R, Chen J-J, Hu B (2015) Transrectal real-time elastography-guided transperineal prostate biopsy as an improved tool for prostate cancer diagnosis. Int J Clin Exp Med 8:6522–6529
Tsutsumi M, Miyagawa T, Matsumura T et al (2007) The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer: clinicopathological analysis. Int J Clin Oncol 12:250–255. https://doi.org/10.1007/s10147-007-0669-7
Kratzenberg J, Salomon G, Tennstedt P et al (2018) Prostate cancer rates in patients with initially negative elastography-targeted biopsy vs. systematic biopsy. World J Urol 36:623–628. https://doi.org/10.1007/s00345-018-2178-x
Bercoff J, Tanter M, Fink M (2004) Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 51:396–409. https://doi.org/10.1109/tuffc.2004.1295425
Boehm K, Salomon G, Beyer B et al (2015) Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J Urol 193:794–800. https://doi.org/10.1016/j.juro.2014.09.100
Woo S, Kim SY, Lee MS et al (2015) Shear wave elastography assessment in the prostate: an intraobserver reproducibility study. Clin Imaging 39:484–487. https://doi.org/10.1016/j.clinimag.2014.11.013
Woo S, Kim SY, Cho JY, Kim SH (2014) Shear wave elastography for detection of prostate cancer: a preliminary study. Korean J Radiol 15:346–355. https://doi.org/10.3348/kjr.2014.15.3.346
Correas J-M, Tissier A-M, Khairoune A et al (2015) Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology 275:280–289. https://doi.org/10.1148/radiol.14140567
Ahmad S, Cao R, Varghese T et al (2013) Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surg Endosc 27:3280–3287. https://doi.org/10.1007/s00464-013-2906-7
Rouvière O, Melodelima C, Hoang Dinh A et al (2017) Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study. Eur Radiol 27:1858–1866. https://doi.org/10.1007/s00330-016-4534-9
Barr RG, Memo R, Schaub CR (2012) Shear wave ultrasound elastography of the prostate: initial results. Ultrasound Q 28:13–20. https://doi.org/10.1097/RUQ.0b013e318249f594
Ji Y, Ruan L, Ren W et al (2019) Stiffness of prostate gland measured by transrectal real-time shear wave elastography for detection of prostate cancer: a feasibility study. Br J Radiol 92:20180970. https://doi.org/10.1259/bjr.20180970
Boehm K, Budäus L, Tennstedt P et al (2015) Prediction of significant prostate cancer at prostate biopsy and per core detection rate of targeted and systematic biopsies using real-time shear wave elastography. Urol Int 95:189–196. https://doi.org/10.1159/000431233
Xiang L-H, Fang Y, Wan J et al (2019) Shear-wave elastography: role in clinically significant prostate cancer with false-negative magnetic resonance imaging. Eur Radiol 29:6682–6689. https://doi.org/10.1007/s00330-019-06274-w
Woo S, Suh CH, Kim SY et al (2017) Shear-wave elastography for detection of prostate cancer: a systematic review and diagnostic meta-analysis. AJR Am J Roentgenol 209:806–814. https://doi.org/10.2214/AJR.17.18056
Yang Y, Zhao X, Zhao X et al (2019) Value of shear wave elastography for diagnosis of primary prostate cancer: a systematic review and meta-analysis. Med Ultrason 21:382–388. https://doi.org/10.11152/mu-2051
Sidhu PS, Cantisani V, Dietrich CF et al (2018) The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med 39:e2–e44. https://doi.org/10.1055/a-0586-1107
Trabulsi EJ, Calio BP, Kamel SI et al (2019) Prostate contrast enhanced transrectal ultrasound evaluation of the prostate with whole-mount prostatectomy correlation. Urology 133:187–191. https://doi.org/10.1016/j.urology.2019.07.026
Roy C, Buy X, Lang H et al (2003) Contrast enhanced color Doppler endorectal sonography of prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedure. J Urol 170:69–72. https://doi.org/10.1097/01.ju.0000072342.01573.8d
Mitterberger MJ, Aigner F, Horninger W et al (2010) Comparative efficiency of contrast-enhanced colour Doppler ultrasound targeted versus systematic biopsy for prostate cancer detection. Eur Radiol 20:2791–2796. https://doi.org/10.1007/s00330-010-1860-1
Halpern EJ, Gomella LG, Forsberg F et al (2012) Contrast enhanced transrectal ultrasound for the detection of prostate cancer: a randomized, double-blind trial of dutasteride pretreatment. J Urol 188:1739–1745. https://doi.org/10.1016/j.juro.2012.07.021
Yunkai Z, Yaqing C, Jun J et al (2019) Comparison of contrast-enhanced ultrasound targeted biopsy versus standard systematic biopsy for clinically significant prostate cancer detection: results of a prospective cohort study with 1024 patients. World J Urol 37:805–811. https://doi.org/10.1007/s00345-018-2441-1
Li Y, Tang J, Fei X, Gao Y (2013) Diagnostic performance of contrast enhanced ultrasound in patients with prostate cancer: a meta-analysis. Acad Radiol 20:156–164. https://doi.org/10.1016/j.acra.2012.09.018
Apfelbeck M, Chaloupka M, Schlenker B et al (2019) Follow-up after focal therapy of the prostate with high intensity focused ultrasound (HIFU) using contrast enhanced ultrasound (CEUS) in combination with MRI image fusion. Clin Hemorheol Microcirc 73:135–143. https://doi.org/10.3233/CH-199222
Moschouris H, Stamatiou K, Malagari K et al (2019) The value of contrast-enhanced ultrasonography in detection of prostatic infarction after prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia. Diagn Interv Radiol Ank Turk 25:134–143. https://doi.org/10.5152/dir.2019.18410
Maxeiner A, Fischer T, Schwabe J et al (1980) (2019) Contrast-enhanced ultrasound (CEUS) and quantitative perfusion analysis in patients with suspicion for prostate cancer. Ultraschall Med Stuttg Ger 40:340–348. https://doi.org/10.1055/a-0594-2093
Kuenen MPJ, Saidov TA, Wijkstra H et al (2013) Spatiotemporal correlation of ultrasound contrast agent dilution curves for angiogenesis localization by dispersion imaging. IEEE Trans Ultrason Ferroelectr Freq Control 60:2665–2669. https://doi.org/10.1109/TUFFC.2013.2865
van Sloun RJ, Demi L, Postema AW et al (2017) Ultrasound-contrast-agent dispersion and velocity imaging for prostate cancer localization. Med Image Anal 35:610–619. https://doi.org/10.1016/j.media.2016.09.010
Postema AW, Frinking PJA, Smeenge M et al (2016) Dynamic contrast-enhanced ultrasound parametric imaging for the detection of prostate cancer. BJU Int 117:598–603. https://doi.org/10.1111/bju.13116
Turco S, Frinking P, Wildeboer R et al (2020) Contrast-enhanced ultrasound quantification: from kinetic modeling to machine learning. Ultrasound Med Biol. https://doi.org/10.1016/j.ultrasmedbio.2019.11.008
Kondo S, Takagi K, Nishida M et al (2017) Computer-aided diagnosis of focal liver lesions using contrast-enhanced ultrasonography with perflubutane microbubbles. IEEE Trans Med Imaging 36:1427–1437. https://doi.org/10.1109/TMI.2017.2659734
Wildeboer RR, Postema AW, Demi L et al (2017) Multiparametric dynamic contrast-enhanced ultrasound imaging of prostate cancer. Eur Radiol 27:3226–3234. https://doi.org/10.1007/s00330-016-4693-8
Palmeri ML, Glass TJ, Miller ZA et al (2016) Identifying clinically significant prostate cancers using 3-D in vivo acoustic radiation force impulse imaging with whole-mount histology validation. Ultrasound Med Biol 42:1251–1262. https://doi.org/10.1016/j.ultrasmedbio.2016.01.004
Zhang M, Tang J, Luo Y et al (2019) Diagnostic performance of multiparametric transrectal ultrasound in localized prostate cancer: a comparative study with magnetic resonance imaging. J Ultrasound Med Off J Am Inst Ultrasound Med 38:1823–1830. https://doi.org/10.1002/jum.14878
Rouvière O, Schoots IG, Mottet N (2019) Multiparametric magnetic resonance imaging before prostate biopsy: a chain is only as strong as its weakest link. Eur Urol 75:889–890. https://doi.org/10.1016/j.eururo.2019.03.023
Wildeboer RR, Mannaerts CK, van Sloun RJG et al (2020) Automated multiparametric localization of prostate cancer based on B-mode, shear-wave elastography, and contrast-enhanced ultrasound radiomics. Eur Radiol 30:806–815. https://doi.org/10.1007/s00330-019-06436-w
Author information
Authors and Affiliations
Contributions
JMC: Project development, Data collection, Manuscript writing. EH: Project development, Manuscript writing. RB: Project development, Manuscript writing. GS: Manuscript writing. JW: Manuscript writing. SB: Data collection, Manuscript writing. CD: Data collection, Manuscript writing. JR: Project development, Manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
JM Correas: Speakers fees: Canon MS, General Electric MS, Hitachi MS, Philips Ultrasound, Siemens Ultrasound, Supersonic Imagine. Equipment grants: Canon MS, Hitachi MS, Philips Ultrasound, SuperSonic Imagine. E Halpern: No potential conflict of interest. R Barr: Project development, Manuscript writing. Speakers fees: Philips Ultrasound, Siemens Ultrasound, Mindray, Canon. Equipment grants: Philips Ultrasound, Siemens Ultrasound, Mindray Ultrasound, SuperSonic Imagine, Canon Ultrasound, Samsung Ultrasound, GE Medical Royalties: Thieme Publishers Advisory panel-Samsung Ultrasound. G Sangeet: No potential conflict of interest. J Walz: No potential conflict of interest. S Bodard: No potential conflict of interest. C Dariane: No potential conflict of interest. J de la Rosette: No potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Correas, JM., Halpern, E.J., Barr, R.G. et al. Advanced ultrasound in the diagnosis of prostate cancer. World J Urol 39, 661–676 (2021). https://doi.org/10.1007/s00345-020-03193-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03193-0