Abstract
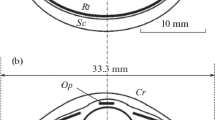
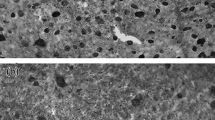
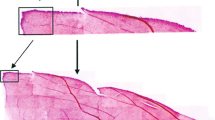
The spatial distribution of retinal ganglion cells provides valuable insight into the importance species place on observing objects in specific regions of their visual field with higher spatial resolving power. We estimate the total number, distribution and peak density of ganglion cells in retinal wholemounts of the sleepy lizard, Tiliqua rugosa, a scincid lizard endemic to southern Australia. Ganglion cells were readily discernable from amacrine cells by their size and shape, prominent nuclei and the accumulation of Nissl-positive substances in their cytoplasm. A total of 1,654,200 (±59,400) presumed ganglion cells were estimated throughout the retina, distributed irregularly and forming a loose horizontal streak of high cell density peaking at 15,500 cells per mm2. With a post nodal distance of 6.25 mm, we calculate an upper limit of visual acuity of 6.8 c/deg.





Similar content being viewed by others
References
Auburn ZM, Bull CM, Kerr GD (2009) The visual perceptual range of a lizard, Tiliqua rugosa. J Ethol 27:75–81
Baker RA, Gawne TJ, Loop MS, Pullman S (2007) Visual acuity of the midland banded water snake estimated from evoked telencephalic potentials. J Comp Physiol A 193:865–870
Barbour HR, Archer MA, Hart NS, Thomas N, Dunlop SA, Beazley LD, Shand J (2002) Retinal characteristics of the ornate dragon lizard, Ctenophorus ornatus. J Comp Neurol 450:334–344
Bartol SM, Musick JA, Ochs AL (2001) Visual acuity thresholds of juvenile loggerhead sea turtles (Caretta caretta): an electrophysiological approach. J Comp Physiol A 187:953–960
Bartol SM, Mellgren RL, Musick JA (2003) Visual acuity of juvenile loggerhead sea turtles (Caretta caretta): a behavioral approach. Int J Comp Psychol 16:143–155
Beazley LD, Sheard PW, Tennant M, Starac D, Dunlop SA (1997) Optic nerve regenerates but does not restore topographic projections in the lizard Ctenophorus ornatus. J Comp Neurol 377:105–120
Bull CM (1987) A population study of the viviparous Australian lizard, Trachydosaurus rugosus (Scincidae). Copeia 1987:749–757
Bull CM (1988) Mate fidelity in an Australian lizard Trachydosaurus rugosus. Behav Ecol Sociobiol 23:45–49
Bull CM (1990) Comparisons of displaced and retained partners in a monogamous lizard. Aust Wildlife Res 17:135–140
Bull CM, Freake MJ (1999) Home range fidelity in the Australian sleepy lizard, Tiliqua rugosa. Aust J Zool 47:125–132
Cogger HG (2000) Reptiles and amphibians of Australia. Reed New Holland, Sydney
Collin SP (1999) Behavioural ecology and retinal cell topography. In: Archer SN, Djamgoz MBS, Loew ER, Partridge JC, Vellarga S (eds) Adaptive mechanisms in the ecology of vision. Kluwer, The Netherlands, pp 509–535
Collin SP (2008) A web-based archive for topographic maps of retinal cell distribution in vertebrates. Clin Exp Optom 91:85–95
Crescitelli F (1972) The visual cells and the visual pigments of the vertebrate eye. In: Dartnall HJA (ed) Handbook of sensory physiology, vol VII/1. Springer, New York, pp 245–363
El Hassni M, Ba M’Hamed S, Reperant J, Bennis M (1997) Quantitative and topographical study of retinal ganglion cells in the chameleon (Chameleo chameleon). Brain Res Bull 44:621–625
Fite K, Lister BC (1981) Bifoveal vision in Anolis lizards. Brain Behav Evol 18:144–154
Fite KV, Rosenfield-Wessels S (1975) A comparative study of deep avian foveas. Brain Behav Evol 12:97–115
Fleishman LJ (1992) The influence of the sensory system and the environment on mption patterns in the visual displays of anoline lizards and other vertebrates. Am Nat 139:S36–S61
Freake MJ (2001) Homing behaviour in the sleepy lizard (Tiliqua rugosa): the role of visual cues and the parietal eye. Behav Ecol Sociobiol 50:563–569
Giorgi PP, Graydon ML (1987) Retinal wholemounts: a simple method for precise mapping. J Neurosci Methods 22:119–124
Gundersen HJG (1977) Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 111:219–223
Gundersen HJG (1986) Stereology of arbitrary particles. J Microsc 143:3–45
Hart NS (2002) Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol 205:3925–3935
Hughes A (1977) The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Autrum H, Jung R, Loewenstein WR, MacKay DM, Teuber HL (eds) Handbook of sensory physiology, vol II/5. Springer, New York, pp 613–756
Kerr GD, Bull CM, Burzacott D (2003) Refuge sites used by the scincid lizard Tiliqua rugosa. Austral Ecol 28:152–161
Makaretz M, Levine L (1980) A light microscopic study on the bifoveate retina in the lizard Anolis carolinensis: general observations and convergence ratios. Vis Res 20:679–686
Murray K, Bull CM (2004) Aggression during monogamous pairing in the sleepy lizard, Tiliqua rugosa: a test of the mate guarding hypothesis. Acta Ethol 7:19–27
Northmore DPM, Granda AM (1991) Refractive state, contrast sensitivity, and resolution in the freshwater turtle, Pseudemys scripta elegans, determined by tectal visual-evoked potentials. Vis Neurosci 7:619–625
Peterson EH (1981) Regional specialization in retinal ganglion cell projection to optic tectum of Dipsosaurus dorsalis (Iguanidae). J Comp Neurol 196:225–252
Pettigrew JD, Dreher B, Hopkins CS, McCall MJ, Brown M (1988) Peak density and distribution of ganglion cells in the retinae of microchiropteran bats: implications for visual acuity. Brain Behav Evol 32:39–56
Pianka ER, Vitt LJ (2003) Lizards: windows to the evolution of diversity. University of California Press, Berkeley
Röll B (2001) Gecko vision—retinal organization, fovea and implications for binocular vision. Vis Res 41:2043–2056
Spellerberg IF (1972) Temperature tolerances of southeast Australian reptiles examined in relation to reptile thermoregulatory behaviour and distribution. Oecologia 9:23–46
Warburg MR (1965) The influence of ambient temperature and humidity on the body temperature and water loss from two Australian lizards, Tiliqua rugosa (Gray) (Scincidae) and Amphibolurus barbants Cuvier (Agamidae). Aust J Zool 13:331–350
Wathey JC, Pettigrew JD (1989) Quantitative analysis of the retinal ganglion cell layer and optic nerve of the barn owl Tyto alba. Brain Behav Evol 33:279–292
Wilhelm M, Straznicky C (1992) The topographic organization of the retinal ganglion cell layer of the lizard Ctenophorus nuchalis. Arch Hist Cytol 55:251–259
Wohlfeil CK (2008) Cues associated with foraging mode in the sleepy lizard, Tiliqua rugosa. BSc Honours thesis, Flinders University of South Australia
Zuri I, Bull CM (2000a) Reduced access to olfactory cues and home-range maintenance in the sleepy lizard (Tiliqua rugosa). J Zool 252:137–145
Zuri I, Bull CM (2000b) The use of visual cues for spatial orientation in the sleepy lizard (Tiliqua rugosa). Can J Zool 78:515–520
Acknowledgments
This work was produced with the assistance of the Australian Research Council under the ARC Centres of Excellence Program. We are grateful to Michelle Lewis and Kerry Gascoigne for providing assistance with histology, and thank Jan Hemmi, Richard Peters and two anonymous reviewers for valuable comments on earlier versions of this manuscript. Experimental procedures were carried out in accordance with guidelines provided by Flinders University of South Australia Animal Welfare Committee in compliance with the Australian Code of Practice for the use of animals for scientific purposes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
New, S.T.D., Bull, C.M. Retinal ganglion cell topography and visual acuity of the sleepy lizard (Tiliqua rugosa). J Comp Physiol A 197, 703–709 (2011). https://doi.org/10.1007/s00359-011-0635-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0635-8