Abstract
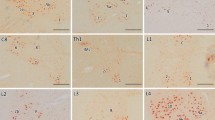
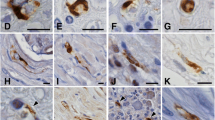
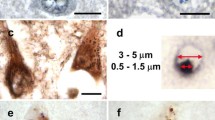
This report concerns an immunohistochemical investigation on RNA-related proteins in the basophilic inclusions (BIs) from patients with adult-onset atypical motor neuron disease. Formalin-fixed, paraffin-embedded sections of the motor cortex and the lumbar spinal cord were examined. The BIs appeared blue in color with H&E and Nissl stain, and pink with methylgreen–pyronin stain. Ribonuclease pretreatment abolished the methylgreen–pyronin staining, suggesting that the BIs contained RNA. Immunohistochemically, the BIs were distinctly labeled with the antibodies against poly(A)-binding protein 1, T cell intracellular antigen 1, and ribosomal protein S6. These proteins are essential constituents of stress granules. In contrast, the BIs were not immunoreactive for ribosomal protein L28 and decapping enzyme 1, which are core components of transport ribonucleoprotein particles and processing bodies, respectively. Moreover, the BIs were not immunopositive for TDP-43. Our results imply that translation attenuation could be involved in the processes of BI formation in this disorder.




Similar content being viewed by others
References
Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115:3227–3234
Anderson P, Kedersha N (2006) RNA granules. J Cell Biol 172:803–808
Andrei MA, Ingelfinger D, Heintzmann R, Achsel T, Rivera-Pomar R, Lührmann R (2005) A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11:717–727
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Bramham CR, Wells DG (2007) Dendric mRNA: transport, translation and function. Nat Rev Neurosci 8:776–789
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Buratti E, Dörk T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20:1774–1784
Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276:36337–36343
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, Consortium for frontotemporal lobar degeneration (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol (Berl) 114:5–22
Hamada K, Fukazawa T, Yanagihara T, Yoshida K, Hamada T, Yoshimura N, Tashiro K (1995) Dementia with ALS features and diffuse Pick body-like inclusions (atypical Pick’s disease?). Clin Neuropathol 14:1–6
Hirano A, Iwata M (1979) Pathology of motor neurons with special reference to amyotrophic lateral sclerosis and related disease. In: Tsubaki T, Toyokura Y (eds) Amyotrophic lateral sclerosis. University of Tokyo Press, Tokyo, pp 107–133
Ishihara K, Araki S, Ihori N, Shiota J, Kawamura M, Nakano I (2006) An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology 26:447–454
Ivanov PA, Nadezhdina ES (2006) Stress granules: RNP-containing cytoplasmic bodies arising in stress: structure and mechanism of organization. Mol Biol 40:844–850
Kedersha NL, Gupta M, Li W, Miller I, Anderson P (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147:1431–1442
Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151:1257–1268
Kiebler MA, Bassell GL (2006) Neuronal RNA granules: movers and makers. Neuron 51:685–690
Krichevsky AM, Kosik KS (2001) Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32:683–696
Kusaka H, Matsumoto S, Imai T (1990) An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol (Berl) 80:660–665
Kusaka H, Matsumoto S, Imai T (1993) Adult-onset motor neuron disease with basophilic intraneuronal inclusion bodies. Clin Neuropathol 12:215–218
Mangus DA, Evans MC, Jacobson A (2003) Poly(A)-binding proteins: multi-functional scaffolds for the post-transcriptional control of gene expression. Genome Biol 4:223–236
Mizuno Y, Amari M, Takatama M, Aizawa H, Mihara B, Okamoto K (2006) Transferrin localizes in Bunina bodies in amyotrophic lateral sclerosis. Acta Neuropathol 112:597–603
Munoz-Garcia D, Ludwin SK (1988) Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructual, and immunocytochemical comparative study. Ann Neurol 16:467–480
Munoz DG (1998) The pathology of pick complex. In: Munoz DG (ed) Pick’s disease and pick complex. Wiley, New York, pp 211–241
Murayama S, Mori H, Ihara Y, Bouldin TW, Suzuki K, Tomonaga M (1990) Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann Neurol 27:137–148
Nakano S, Shinde A, Ito H, Ito H, Kusaka H (2005) Messenger RNA degradation may be inhibited in sporadic inclusion body myositis. Neurology 65:420–425
Nelson JS, Prensky AL (1972) Sporadic juvenile amyotrophic lateral sclerosis. a clinic-pathological study of a case with neuronal cytoplasmic inclusions containing RNA. Arch Neurol 27:300–306
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Oda M, Akagawa N, Tabuchi Y, Tanabe H (1978) A sporadic juvenile case of the amyotrophic lateral sclerosis with neuronal intracytoplasmic inclusions. Acta Neuropathol (Berl) 44:211–216
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Parker R, Sheth U (2007) P bodies and the control of mRNA translation and degradation. Mol Cell 25:635–646
Sasaki S, Toi S, Shirata A, Yamane K, Sakuma H, Iwata M (2001) Immunohistochemical and ultrastructual study of basophilic inclusions in adult-onset motor neuron disease. Acta Neuropathol (Berl) 102:200–206
Wang IF, Wu LS, Chang HY, Shen CK (2008) TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem 105:797–806
Yokota O, Tsuchiya K, Terada S, Ishizu H, Uchikado H, Ikeda M, Oyanagi K, Nakano I, Murayama S, Kuroda S, Akiyama H (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Acknowledgment
We express our sincere appreciation to Miss Tomoko Takemi for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, K., Ito, H., Nakano, S. et al. Immunohistochemical identification of messenger RNA-related proteins in basophilic inclusions of adult-onset atypical motor neuron disease. Acta Neuropathol 116, 439–445 (2008). https://doi.org/10.1007/s00401-008-0415-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0415-x