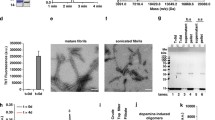
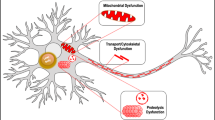
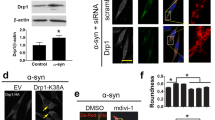
Abstract
The accumulation of misfolded α-synuclein (aSyn) and neuron loss define several neurodegenerative disorders including Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). However, the precise relationship between pathology and neurotoxicity and why these processes disproportionately affect certain neuron subpopulations are poorly understood. We show here that Math2-expressing neurons in the hippocampal Cornu ammonis (CA), a region significantly affected by aSyn pathology in advanced PD and DLB, are highly susceptible to pathological seeding with pre-formed fibrils (PFFs), in contrast to dentate gyrus neurons, which are relatively spared. Math2+ neurons also exhibited more rapid and severe cell loss in both in vitro and in vivo models of synucleinopathy. Toxicity resulting from PFF exposure was dependent on endogenous aSyn and could be attenuated by N-acetyl-cysteine through a glutathione-dependent process. Moreover, aSyn expression levels strongly correlate with relative vulnerability among hippocampal neuron subtypes of which Math2+ neurons contained the highest amount. Consistent with this, antisense oligonucleotide (ASO)-mediated knockdown of aSyn reduced the neuronal pathology in a time-dependent manner. However, significant neuroprotection was observed only with early ASO intervention and a substantial reduction of aSyn pathology, indicating toxicity occurs after a critical threshold of pathological burden is exceeded in vulnerable neurons. Together, our findings reveal considerable heterogeneity in endogenous aSyn levels among hippocampal neurons and suggest that this may contribute to the selective vulnerability observed in the context of synucleinopathies.








Similar content being viewed by others
Abbreviations
- aSyn:
-
Alpha-synuclein
- pSyn:
-
p-Ser129 alpha-synuclein
- ASO:
-
Antisense oligonucleotide
- CA:
-
Cornu ammonis
- DG:
-
Dentate gyrus
- DPI:
-
Days post-injection
- DPT:
-
Days post-transduction
- PD:
-
Parkinson’s disease
- DLB:
-
Dementia with Lewy bodies
- PDD:
-
Parkinson’s disease with dementia
- PFF:
-
Pre-formed fibril
- LB/LN:
-
Lewy body/Lewy neurite
- Ms:
-
Mouse
- Hu:
-
Human
- SNpc:
-
Substantia nigra pars compacta
- wt:
-
Wildtype
References
Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB, Moehle MS, Stoyka L, Anabtawi N, Krendelchtchikova V et al (2017) alpha-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiol Dis 105:84–98. https://doi.org/10.1016/j.nbd.2017.05.014
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252
Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, Gage FH (2017) Hippocampal alpha-synuclein in dementia with lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 37:1675–1684. https://doi.org/10.1523/JNEUROSCI.3047-16.2016
Ansorge O, Daniel SE, Pearce RK (1997) Neuronal loss and plasticity in the supraoptic nucleus in Parkinson’s disease. Neurology 49:610–613
Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD (2005) Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221. https://doi.org/10.1016/j.neuron.2004.12.036
Armstrong RA, Kotzbauer PT, Perlmutter JS, Campbell MC, Hurth KM, Schmidt RE, Cairns NJ (2014) A quantitative study of alpha-synuclein pathology in fifteen cases of dementia associated with Parkinson disease. J Neural Transm (Vienna) 121:171–181. https://doi.org/10.1007/s00702-013-1084-z
Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ (2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129:4249–4260
Barral S, Cheng R, Reitz C, Vardarajan B, Lee J, Kunkle B, Beecham G, Cantwell LS, Pericak-Vance MA, Farrer LA et al (2015) Linkage analyses in Caribbean Hispanic families identify novel loci associated with familial late-onset Alzheimer’s disease. Alzheimer’s Dement 11:1397–1406. https://doi.org/10.1016/j.jalz.2015.07.487
Baxter KK, Uittenbogaard M, Chiaramello A (2012) The neurogenic basic helix-loop-helix transcription factor NeuroD6 enhances mitochondrial biogenesis and bioenergetics to confer tolerance of neuronal PC12-NeuroD6 cells to the mitochondrial stressor rotenone. Exp Cell Res 318:2200–2214. https://doi.org/10.1016/j.yexcr.2012.07.004
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R et al (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634. https://doi.org/10.1007/s00401-009-0538-8
Bilsland J, Roy S, Xanthoudakis S, Nicholson DW, Han Y, Grimm E, Hefti F, Harper SJ (2002) Caspase inhibitors attenuate 1-methyl-4-phenylpyridinium toxicity in primary cultures of mesencephalic dopaminergic neurons. J Neurosci 22:2637–2649 (20026227)
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA (2003) Parkinson’s disease is associated with hippocampal atrophy. Mov Disord 18:784–790. https://doi.org/10.1002/mds.10444
Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N (2016) Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 5:e14997. https://doi.org/10.7554/elife.14997
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M et al (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. https://doi.org/10.1016/S0140-6736(04)17103-1
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F et al (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328. https://doi.org/10.1126/science.1129462
Cosi C, Colpaert F, Koek W, Degryse A, Marien M (1996) Poly(ADP-ribose) polymerase inhibitors protect against MPTP-induced depletions of striatal dopamine and cortical noradrenaline in C57B1/6 mice. Brain Res 729:264–269
Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA et al (2012) Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell 149:1048–1059. https://doi.org/10.1016/j.cell.2012.03.037
Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA et al (2002) Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci 99:14524–14529. https://doi.org/10.1073/pnas.172514599
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci 106:13010–13015. https://doi.org/10.1073/pnas.0903691106
Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, Burnett M, Parisi JE, Klos KJ, Ahlskog JE (2009) Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15(Suppl 3):S1–S5. https://doi.org/10.1016/S1353-8020(09)70769-2
Doorn KJ, Moors T, Drukarch B, van de Berg W, Lucassen PJ, van Dam AM (2014) Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun 2:90. https://doi.org/10.1186/s40478-014-0090-1
Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT, Surmeier DJ (2013) Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci 33:10154–10164. https://doi.org/10.1523/JNEUROSCI.5311-12.2013
Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D et al (2004) Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol 55:174–179. https://doi.org/10.1002/ana.10846
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N (2013) Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61:349–360. https://doi.org/10.1002/glia.22437
Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G et al (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci 102:3413–3418. https://doi.org/10.1073/pnas.0409713102
Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW (1992) Astrocytes and Parkinson’s disease. Prog Brain Res 94:429–436
Fowler KD, Funt JM, Artyomov MN, Zeskind B, Kolitz SE, Towfic F (2015) Leveraging existing data sets to generate new insights into Alzheimer’s disease biology in specific patient subsets. Sci Rep 5:14324. https://doi.org/10.1038/srep14324
Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M et al (2007) Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 68:916–922. https://doi.org/10.1212/01.wnl.0000254458.17630.c5
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. https://doi.org/10.1038/ncb748
Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D (2014) Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 137:2493–2508. https://doi.org/10.1093/brain/awu193
Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB (2017) alpha-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun 5:85. https://doi.org/10.1186/s40478-017-0494-9
Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M et al (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci 97:2875–2880. https://doi.org/10.1073/pnas.040556597
Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, Iwaki T, Ohara T, Sasaki T, LaFerla FM et al (2014) Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb Cortex 24:2476–2488. https://doi.org/10.1093/cercor/bht101
Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D (2013) In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord 19:47–52. https://doi.org/10.1016/j.parkreldis.2012.07.002
Jenner P (2008) Functional models of Parkinson’s disease: a valuable tool in the development of novel therapies. Ann Neurol 64(Suppl 2):S16–S29. https://doi.org/10.1002/ana.21489
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51. https://doi.org/10.1038/nature12481
Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ et al (2013) Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562. https://doi.org/10.1038/ncomms2534
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136:2419–2431. https://doi.org/10.1093/brain/awt192
Kremer HP, Bots GT (1993) Lewy bodies in the lateral hypothalamus: do they imply neuronal loss? Mov Disord 8:315–320. https://doi.org/10.1002/mds.870080310
Lee VM, Trojanowski JQ (2006) Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron 52:33–38. https://doi.org/10.1016/j.neuron.2006.09.026
Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, Lee BD, Kang HC et al (2013) Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci 16:1392–1400. https://doi.org/10.1038/nn.3500
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ et al (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176. https://doi.org/10.1038/nature05453
Li X, Long J, He T, Belshaw R, Scott J (2015) Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer’s disease. Sci Rep 5:12393. https://doi.org/10.1038/srep12393
Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD, Pitkin RM, Decker SC, Trojanowski JQ, Lee VM (2016) Molular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep 16:3373–3387. https://doi.org/10.1016/j.celrep.2016.08.053
Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953. https://doi.org/10.1126/science.1227157
Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209:975–986. https://doi.org/10.1084/jem.20112457
Mahul-Mellier AL, Vercruysse F, Maco B, Ait-Bouziad N, De Roo M, Muller D, Lashuel HA (2015) Fibril growth and seeding capacity play key roles in alpha-synuclein-mediated apoptotic cell death. Cell Death Differ 22:2107–2122. https://doi.org/10.1038/cdd.2015.79
Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M (2014) Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun 2:88. https://doi.org/10.1186/s40478-014-0088-8
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M (2013) Prion-like spreading of pathological alpha-synuclein in brain. Brain 136:1128–1138. https://doi.org/10.1093/brain/awt037
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK (2015) Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep 10:1252–1260. https://doi.org/10.1016/j.celrep.2015.01.060
Ostergaard ME, Southwell AL, Kordasiewicz H, Watt AT, Skotte NH, Doty CN, Vaid K, Villanueva EB, Swayze EE, Bennett CF et al (2013) Rational design of antisense oligonucleotides targeting single nucleotide polymorphisms for potent and allele selective suppression of mutant Huntingtin in the CNS. Nucleic Acids Res 41:9634–9650. https://doi.org/10.1093/nar/gkt725
Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA et al (2012) dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337:481–484. https://doi.org/10.1126/science.1223899
Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE (2015) Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol 25:2349–2360. https://doi.org/10.1016/j.cub.2015.07.050
Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E et al (2015) Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis 82:185–199. https://doi.org/10.1016/j.nbd.2015.06.003
Peterson C, Neal JH, Cotman CW (1989) Development of N-methyl-D-aspartate excitotoxicity in cultured hippocampal neurons. Brain Res Dev Brain Res 48:187–195
Poulopoulos M, Levy OA, Alcalay RN (2012) The neuropathology of genetic Parkinson’s disease. Mov Disord 27:831–842. https://doi.org/10.1002/mds.24962
Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C et al (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75:351–362. https://doi.org/10.1002/ana.24066
Satoh J, Yamamoto Y, Asahina N, Kitano S, Kino Y (2014) RNA-Seq data mining: downregulation of NeuroD6 serves as a possible biomarker for alzheimer’s disease brains. Dis Mark 2014:123165. https://doi.org/10.1155/2014/123165
Seth PP, Yu J, Allerson CR, Berdeja A, Swayze EE (2011) Synthesis and biophysical characterization of R-6′-Me-alpha-L-LNA modified oligonucleotides. Bioorg Med Chem Lett 21:1122–1125. https://doi.org/10.1016/j.bmcl.2010.12.119
Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M, Higuchi M, Yanai K, Hisanaga SI, Hasegawa M (2017) Propagation of pathological alpha-synuclein in marmoset brain. Acta Neuropathol Commun 5:12. https://doi.org/10.1186/s40478-017-0413-0
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R et al (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. https://doi.org/10.1126/science.1090278
Sugiyama T, Osumi N, Katsuyama Y (2014) A novel cell migratory zone in the developing hippocampal formation. J Comp Neurol 522:3520–3538. https://doi.org/10.1002/cne.23621
Sulzer D, Surmeier DJ (2013) Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord 28:41–50. https://doi.org/10.1002/mds.25095
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. https://doi.org/10.1038/nrn.2016.178
Taguchi K, Watanabe Y, Tsujimura A, Tatebe H, Miyata S, Tokuda T, Mizuno T, Tanaka M (2014) Differential expression of alpha-synuclein in hippocampal neurons. PLoS One 9:e89327. https://doi.org/10.1371/journal.pone.0089327
Tapias V, Hu X, Luk KC, Sanders LH, Lee VM, Greenamyre JT (2017) Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci 74:2851–2874. https://doi.org/10.1007/s00018-017-2541-x
Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, Lee VMY, Trojanowski JQ, Bjorklund A (2017) Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci 114:E8284–E8293. https://doi.org/10.1073/pnas.1710442114
Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, Sedor S, Waits K, Boluda S, Grossman M, Van Deerlin VM et al (2016) Pathological alpha-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathol 131:393–409. https://doi.org/10.1007/s00401-015-1526-9
Trinh J, Farrer M (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9:445–454. https://doi.org/10.1038/nrneurol.2013.132
Uittenbogaard M, Baxter KK, Chiaramello A (2010) The neurogenic basic helix-loop-helix transcription factor NeuroD6 confers tolerance to oxidative stress by triggering an antioxidant response and sustaining the mitochondrial biomass. ASN Neuro 2:e00034. https://doi.org/10.1042/AN20100005
Uittenbogaard M, Chiaramello A (2005) The basic helix-loop-helix transcription factor Nex-1/Math-2 promotes neuronal survival of PC12 cells by modulating the dynamic expression of anti-apoptotic and cell cycle regulators. J Neurochem 92:585–596. https://doi.org/10.1111/j.1471-4159.2004.02886.x
Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE (2016) Alpha-synuclein-based animal models of parkinson’s disease: challenges and opportunities in a New Era. Trends Neurosci 39:750–762. https://doi.org/10.1016/j.tins.2016.09.003
Volles MJ, Lansbury PT Jr (2002) Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41:4595–4602
Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM (2014) Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 25:4010–4023. https://doi.org/10.1091/mbc.E14-02-0741
Volpicelli-Daley LA, Luk KC, Lee VM (2014) Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9:2135–2146. https://doi.org/10.1038/nprot.2014.143
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM (2011) Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72:57–71. https://doi.org/10.1016/j.neuron.2011.08.033
Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, Guo M, Wilson B, Hong JS, Zhang J (2015) alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci 112:E1926–E1935. https://doi.org/10.1073/pnas.1417883112
Withers GS, George JM, Banker GA, Clayton DF (1997) Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res 99:87–94
Wong YC, Krainc D (2017) alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23:1–13. https://doi.org/10.1038/nm.4269
Yang W, Yu S (2017) Synucleinopathies: common features and hippocampal manifestations. Cell Mol Life Sci 74:1485–1501. https://doi.org/10.1007/s00018-016-2411-y
Acknowledgements
We thank Drs. Xiaolu Yang, Michael Henderson, Dustin Covell, Chao Peng, Kurt Brunden, and John Trojanowski for helpful discussions and insights. This research was funded in part by the NIH grants NS088322, NS053488, T32-AG000255, and a pilot grant from University of Pennsylvania Institute for Translational Medicine and Therapeutics. T. C. was an employee of Ionis Pharmaceuticals at the time when data were generated and interpreted. The remaining authors have no additional financial interests.
Author information
Authors and Affiliations
Contributions
Conceptualization, EL and KCL; Methodology, EL, AC, BZ, SCD, DMR, and KCL; Investigation, EL, CC, SX, and KCL; Writing—original draft, EL; Writing—review and editing, EL, VMYL and KCL; Funding Acquisition, KCL, VMYL; Resources, KCL; Supervision, KCL.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luna, E., Decker, S.C., Riddle, D.M. et al. Differential α-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol 135, 855–875 (2018). https://doi.org/10.1007/s00401-018-1829-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1829-8