Abstract
Purpose
To compare symptomatic response in Indian women using different estrogen preparations for treatment of menopausal symptoms.
Methodology
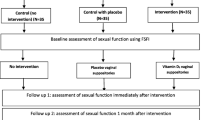
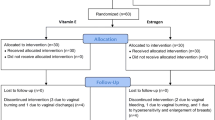
A randomized, single blind, four arm, parallel assignment study was conducted in VMMC and SJH, New Delhi, India. 200 Indian menopausal women were recruited and assigned into four treatment groups viz., estradiol valerate (E2V), conjugated equine estrogen (CEE), isoflavones and Placebo group. The statistical significance of categorical variables was determined by Chi-square, Fisher’s exact test. In case of quantitative variable parametric test Student’s t test was used. In case of quantitative variables where data are not normally distributed, Kruskal–wallis test and Wilcoxon Mann–Whitney test were used. Symptomatic response in vasomotor/vaginal symptoms was assessed in all groups.
Results
Both E2V and CEE groups were effective in reducing severity and frequency of hot flashes. 91.9 % decrease was observed in mean hot flash score in the E2V group after 24 weeks of treatment, 89.2 % in the CEE group, 60.42 % decrease in the isoflavones group. While placebo led to 47.9 % decrease in mean hot flash score. After 24 weeks of therapy there was significant increase in vaginal health index in the E2V and CEE and the isoflavones group. No serious side effect was reported in any of the groups.
Conclusion
Low doses of both CEE and E2V were equally effective for management of vasomotor/vaginal symptoms when administered over 24 weeks. However, it seems more reasonable to replenish with less costly and bio-identical hormone, i.e. micronized estradiol valerate which is equally effective.
Trial registry
The trial was registered under Clinical trial registry of India prospectively (number: CTRI/2012/04/002566).


Similar content being viewed by others
References
American College of Obstetricians and Gynecologists (2014) ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol 123:202–216
Grady D (2006) Clinical practice. Management of menopausal symptoms. N Engl J Med 355:2338–2347
Randolph JF Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT et al (2005) The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 90:6106–6112
Mishra G, Kok H, Ecob R, Cooper R, Hardy R, Kuh D (2006) Cessation of hormone replacement therapy after reports of adverse findings from randomized controlled trials: evidence from a british birth cohort. Am J Public Health 96(7):1219–1225
Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J (2008) Long-term impact of the women’s health initiative on HRT. Arch Gynecol Obstet 277(3):219–224
Ettinger B, Wang SM, Leslie RS, Patel BV, Boulware MJ, Mann ME, McBride M (2012) Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 19(6):610–615
Barbaglia G, Macia` F, Comas M, Sala M, Del Mar Vernet M, Casamitjana M et al (2009) Trends in hormone therapy use before and after publication of the women’s health initiative trial: 10 years of follow-up. Menopause 16(5):1061–1064
Bilgrami I, Blower K, Feng J, Stefanko G, Tan E (2004) Changes in the use of hormone replacement therapy in New Zealand following the publication of the Women’s Health Initiative trial. N Z Med J 117(1206):U1175
Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ (2012) A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the women’s health initiative. Menopause 2012(19):616–621
Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM et al (2007) Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477
Honjo H, Taketani Y (2009) Low-dose estradiol for climacteric symptoms in Japanese women: a randomized, controlled trial. Climacteric 12:319–328
Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J (2000) Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol 95:726–731
Chaumeli JC (1998) Micronization: a method of improving bioavailability of poorly soluble drugs. Methods Find Exp Clin Pharmacol 20:211
Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J (2007) Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev (4):CD001395
Stearns V, Loprinzi CL (2003) New therapeutic approaches for hot flashes in women. J Support Oncol 1:11–21
Sharma P, Goel N, Rajaram S, Sharma A, Agarwal N (2006) Assessment of modified vaginal health index in postmenopausal women on micronized 17 estradiol therapy. J obstet Gynaecol India 56(5):434–437
Chattha R, Kulkarni R, Nagarathna R, Nagendra HR (2008) Factor analysis of Greene’s Climcateric scale for Indian women. Maturitas 59:22–27
Greendale GA, Reboussin BA, Hogan P, Barnabei VM, Shumaker S, Johnson S et al (1998) Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 92:982–988
Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH (2001) Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 75:1065–1079
Johnson SR, Ettinger B, Macer JL, Ensrud KE, Quan J, Grady D (2005) Uterine and vaginal effects of unopposed ultralow-dose transdermal estradiol. Obstet Gynecol 105:779–787
Long CY, Liu CM, Hsu SC, Wu CH, Wang CL, Tsai EM (2006) A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause 13(5):737–743
Pines A, Sturdee DW, Maclennan AH, Schneider HP, Burger H, Fenton A (2007) The heart of the WHI study: time for hormone therapy policies to be revised. Climacteric 10:267–269
Zhang F, Chen Y, Pisha E, Shen L, Xiong Y, van Breeman RB, Bolton JL (1999) The major metabolite of equilin, 4-hydroxyequilin, autooxidizestoano-quinone which isomerizes to the potent cytotoxin 4-hydroxyequilenin-o-quinone. Chem Res Toxicol 12:204–213
Barton M, Dubey RK (2002) Postmenopausal hormone-replacement therapy. N Engl J Med 346:63–65
Lippert TH, Mueck AO, Seeger H (2000) Is the use of conjugated equine oestrogens in hormone replacement therapy still appropriate? Br J Clin Pharmacol 49(5):489–490
Panay N, Hamoda H, Arya R, Sawas M, on behalf of The British Menopause Society and Women’s Health Concern (2013) The 2013 british menopause society & women’s heath concern recommendations on hormone replacement therapy. Menopause Int. doi:10.1177/1754045313489645
Bærug U, Winge T, Nordland E, Faber-Swensson E, Heldaas K, Norling B et al (1998) Do combinations of 1 mg estradiol and low doses of NETA effectively control menopausal symptoms? Climacteric 1:219–228
Mirkin S, Amadio JM, Bernick BA, Pickar JH, Archer DF (2015) 17β-Estradiol and natural progesterone for menopausal hormone therapy: REPLENISH phase 3 study design of a combination capsule and evidence review. Maturitas 81(1):28–35
Bingol B, Gunenc Z, Yilmaz M, Biri A, Tiras B, Güner H (2010) Effects of hormone replacement therapy on glucose and lipid profiles and on cardiovascular risk parameters in postmenopausal women. Arch Gynecol Obstet 281(5):857–864
Windler E, Stute P, Ortmann O, Mueck AO (2015) Is postmenopausal hormone replacement therapy suitable after a cardio- or cerebrovascular event? Arch Gynecol Obstet 291(1):213–217
Acknowledgments
The authors would like to acknowledge all the women who participated in the study and the staff of Safdarjung Hospital for their support. Authors would also like to acknowledge Walter Bushnell Laboratories for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Funding
Funding was provided by Walter Bushnell Laboratories.
Ethics approval
Institutional ethics committee approved the project. No: 47-11/EC (5/81), dated 13/10/12.
Rights and permissions
About this article
Cite this article
Malik, S., Pannu, D., Prateek, S. et al. Comparison of the symptomatic response in Indian menopausal women with different estrogen preparations for the treatment of menopausal symptoms: a randomized controlled trial. Arch Gynecol Obstet 293, 1325–1333 (2016). https://doi.org/10.1007/s00404-016-4034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4034-9