Abstract
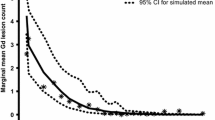
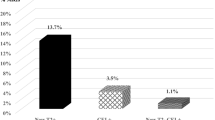
The objective of this study is to characterize the timing and extent of radiologic MS disease recurrence during the 24-week natalizumab treatment interruption period in RESTORE. RESTORE was a randomized, partially placebo-controlled exploratory study. Natalizumab-treated patients with no gadolinium-enhancing (Gd+) lesions at screening (n = 175) were randomized 1:1:2 to continue natalizumab (n = 45), switch to placebo (n = 42), or switch to other therapies (n = 88) for 24 weeks. MRI assessments were performed every 4 weeks. Predictors of increased numbers of Gd+ lesions during natalizumab treatment interruption were evaluated. The numbers of Gd+ lesions were compared with retrospectively collected pre-natalizumab MRI reports and data from placebo-treated patients from two historical randomized clinical trials. Gd+ lesions were detected in 0 % (0/45) of natalizumab patients, 61 % (25/41) of placebo patients, and 48 % (39/81) of other-therapies patients during the randomized treatment period. Gd+ lesions were detected starting at week 12; most were observed at week 16 or later. Thirteen percent (14/107) of patients had >5 Gd+ lesions on ≥1 (of 6) scans during the randomized treatment period versus 7 % (7/107) of patients pre-natalizumab (based on medical record of a single scan). Younger patients and those with more Gd+ lesions pre-natalizumab were more likely to have increased MRI activity. Distribution of total and persistent Gd+ lesions in RESTORE patients was similar to placebo-treated historical control patients. In most patients, recurring radiological disease activity during natalizumab interruption did not exceed pre-natalizumab levels or levels seen in historical control patients.




Similar content being viewed by others
References
Belachew S, Phan-Ba R, Bartholomé E, Delvaux V, Hansen I, Calay P, Hafsi KE, Moonen G, Tshibanda L, Vokaer M (2011) Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing-remitting multiple sclerosis. Eur J Neurol 18:240–245
Putzki N, Yaldizli O, Maurer M, Cursiefen S, Kuckert S, Klawe C, Maschke M, Tettenborn B, Limmroth V (2010) Efficacy of natalizumab in second line therapy of relapsing-remitting multiple sclerosis: results from a multi-center study in German speaking countries. Eur J Neurol 17:31–37
Hutchinson M, Kappos L, Calabresi PA, Confavreux C, Giovannoni G, Galetta SL, Havrdova E, Lublin FD, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, AFFIRM and SENTINEL Investigators (2009) The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 256:405–415
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366:1870–1880
Sorensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E, Kappos L, Kieseier BC, Montalban X, Olsson T (2012) Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 18:143–152
Borriello G, Prosperini L, Mancinelli C, Gianni C, Fubelli F, Pozzilli C (2012) Pulse monthly steroids during an elective interruption of natalizumab: a post-marketing study. Eur J Neurol 19:783–787
Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C (2011) Observations during an elective interruption of natalizumab treatment: a post-marketing study. Mult Scler 17:372–375
Kaufman MD, Lee R, Norton HJ (2011) Course of relapsing-remitting multiple sclerosis before, during and after natalizumab. Mult Scler 17:490–494
Killestein J, Vennegoor A, Strijbis EM, Seewann A, van Oosten BW, Uitdehaag BM, Polman CH (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68:392–395
West TW, Cree BA (2010) Natalizumab dosage suspension: are we helping or hurting? Ann Neurol 68:395–399
Magraner MJ, Coret F, Navarre A, Bosca I, Simo M, Escutia M, Bernad A, Navarro L, Casanova B (2011) Pulsed steroids followed by glatiramer acetate to prevent inflammatory activity after cessation of natalizumab therapy: a prospective, 6-month observational study. J Neurol 258:1805–1811
Havla J, Gerdes LA, Meinl I, Krumbholz M, Faber H, Weber F, Pellkofer HL, Hohlfeld R, Kumpfel T (2011) De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669
Rossi S, Motta C, Studer V, De Chiara V, Barbieri F, Monteleone F, Fornasiero A, Coarelli G, Bernardi G, Cutter G, Stuve O, Salvetti M, Centonze D (2013) Effect of glatiramer acetate on disease reactivation in MS patients discontinuing natalizumab. Eur J Neurol 20:87–94
Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, Desormeaux C, Guiziou C, Kassiotis P, Lallement F, Laplaud D, Diraison P, Rouhart F, Sartori E, Wardi R, Wiertlewski S, Edan G (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308:98–102
Miravalle A, Jensen R, Kinkel RP (2011) Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 68:186–191
O’Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C, Rudick RA, Aschenbach W, Lucas N (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76:1858–1865
Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH (2008) Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 70:1150–1151
Rinaldi F, Seppi D, Calabrese M, Perini P, Gallo P (2012) Switching therapy from natalizumab to fingolimod in relapsing-remitting multiple sclerosis: clinical and magnetic resonance imaging findings. Mult Scler 18:1640–1643
Sempere AP, Martin-Medina P, Berenguer-Ruiz L, Perez-Carmona N, Sanchez-Perez R, Polache-Vengud J, Feliu-Rey E (2013) Switching from natalizumab to fingolimod: an observational study. Acta Neurol Scand 128:e6–e10
Rigau V, Mania A, Befort P, Carlander B, Jonquet O, Lassmann H, Camu W, Thouvenot E (2012) Lethal multiple sclerosis relapse after natalizumab withdrawal. Neurology 79:2214–2216
Lenhard T, Biller A, Mueller W, Metz I, Schonberger J, Wildemann B (2010) Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology 75:831–833
Hellwig K, Gold R (2011) Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology 76:1362–1363
Stüve O, Cravens PD, Frohman EM, Phillips JT, Remington GM, von Geldern G, Cepok S, Singh MP, Tervaert JW, De Baets M, MacManus D, Miller DH, Radu EW, Cameron EM, Monson NL, Zhang S, Kim R, Hemmer B, Racke MK (2009) Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology 72:396–401
Fox RJ, Cree B, De Seze J, Gold R, Hartung H-P, Jeffery D, Kappos L, Kaufman M, Montalban X, Weinstock-Guttman B, Anderson B, Natarajan A, Ticho B, Duda P (2014) MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology 82:1491–1498
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW (2003) A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 348:15–23
Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, O’Neill GN (2008) Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372(9648):1463–1472
Centonze D, Rossi S, Rinaldi F, Gallo P (2012) Severe relapses under fingolimod treatment prescribed after natalizumab. Neurology 79:2004–2005
Tubridy N, Behan PO, Capildeo R, Chaudhuri A, Forbes R, Hawkins CP, Hughes RA, Palace J, Sharrack B, Swingler R, Young C, Moseley IF, Macmanus DG, Donoghue S, Miller DH (1999) The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology 53:466–472
Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A (2012) Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol 19:1060–1069
Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdova E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O’Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radu EW, Sorensen PS, King J (2011) Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 10:745–758
Dong-Si T, Richman S, Bloomgren G, Wenten M, Philip J, Datta S, McIninch M, Bozic C, Ticho B, Richert N (2013) Survival and functional outcome in asymptomatic natalizumab-associated progressive multifocal leukoencephalopathy multiple sclerosis patients. Mult Scler 19(suppl 1):397 (P879)
Acknowledgments
Biogen Idec provided funding for editorial support in the development of this paper; Britt Anderson, PhD, from Infusion Communications wrote the first draft of the manuscript based on input from authors, and Josh Safran and Jackie Cannon from Infusion Communications copyedited and styled the manuscript per journal requirements. Biogen Idec reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content. This study was funded by Biogen Idec Inc.
Conflicts of interest
Dr. Kaufman has received honoraria and research support from Biogen Idec, has received financial support from Bayer, EMD Serono, Novartis, and Teva, and is a consultant for Department of Defense. Dr. Cree has received consulting honoraria from AbbVie, Biogen Idec, EMD Serono, Genzyme, Novartis, Sanofi, and Teva Neurosciences. Dr. De Sèze has received honoraria from Bayer Schering, Biogen Idec, LFB, Merck Serono, Novartis, Sanofi, and Teva. Dr. Fox has received consultant fees from Allozyne, Avanir, Biogen Idec, EMD Serono, Novartis, Questcor, Teva, and Xenoport and has received research support from Biogen Idec and Novartis. Dr. Gold has received research support and honoraria from Bayer HealthCare, Biogen Idec, Merck Serono, and Teva, is Editor-in-Chief of Therapeutic Advances in Neurological Disorders, has received a license fee from Biogen Idec (no future rights), and has received research support from Bayer HealthCare, Biogen Idec, Merck Serono, Novartis, and Teva. Dr. Hartung has received honoraria for consulting and speaking at symposia from Bayer HealthCare, Biogen Idec, BioMS, Genzyme, Merck Serono, Novartis, Roche, Sanofi, and Teva. Dr. Jeffery is a consultant for Acorda, Bayer HealthCare, EMD Serono, Genzyme, GlaxoSmithKline, Novartis, Pfizer, Questcor, and Teva, and has received research support from Berlex, Biogen Idec, Novartis, Pfizer, and Teva. Dr. Kappos has received research support from Acorda, Actelion, Allozyne, BaroFold, Bayer HealthCare, Bayer Schering, Bayhill, Biogen Idec, Boehringer Ingelheim, Elan, Genmab, GlaxoSmithKline, Glenmark, Merck Serono, MediciNova, Novartis, Sanofi, Santhera, Shire, Roche, Teva, UCB, Wyeth, the Swiss MS Society, the Swiss National Research Foundation, the European Union, the Gianni Rubatto Foundation, and the Novartis and Roche Research Foundations. Dr. Montalbán has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials, or has participated in advisory boards of clinical trials in the past years with Almirall, Bayer Schering, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi, and Teva. Dr. Weinstock-Guttman has received honoraria for speaking and serving on scientific advisory boards from Acorda, Biogen Idec, EMD Serono, Novartis, Pfizer, and Teva and has received financial support for research from Acorda, Biogen Idec, EMD Serono, Novartis, Pfizer, and Teva. Drs. Duda and Ticho were employees of Biogen Idec at the time of the study and manuscript preparation. Drs. Pace and Campagnolo are employees of Biogen Idec.
Ethical standard
Each site’s institutional review board reviewed and approved the study protocol and amendments, and all participants provided written informed consent. The RESTORE study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guideline on Good Clinical Practice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Ticho and Duda are former employees of Biogen Idec Inc. and were at the company during study conduct.
Rights and permissions
About this article
Cite this article
Kaufman, M., Cree, B.A.C., De Sèze, J. et al. Radiologic MS disease activity during natalizumab treatment interruption: findings from RESTORE. J Neurol 262, 326–336 (2015). https://doi.org/10.1007/s00415-014-7558-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7558-6