Abstract
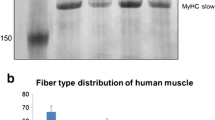
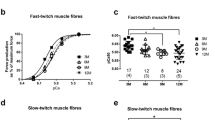
Adult human jaw muscles differ from limb and trunk muscles in enzyme-histochemical fibre type composition. Recently, we showed that the human masseter and biceps differ in fibre type pattern already at childhood. The present study explored the myosin heavy-chain (MyHC) expression in the young masseter and biceps muscles by means of gel electrophoresis (GE) and immuno-histochemical (IHC) techniques. Plasticity in MyHC expression during life was evaluated by comparing the results with the previously reported data for adult muscles. In young masseter, GE identified MyHC-I, MyHC-IIa MyHC-IIx and small proportions of MyHC-fetal and MyHC-α cardiac. Western blots confirmed the presence of MyHC-I, MyHC-IIa and MyHC-IIx. IHC revealed in the masseter six isomyosins, MyHC-I, MyHC-IIa, MyHC-IIx, MyHC-fetal, MyHC α-cardiac and a previously not reported isoform, termed MyHC-IIx′. The majority of the masseter fibres co-expressed two to four isoforms. In the young biceps, both GE and IHC identified MyHC-I, MyHC-IIa and MyHC-IIx. MyHC-I predominated in both muscles. Young masseter showed more slow and less-fast and fetal MyHC than the adult and elderly masseter. These results provide evidence that the young masseter muscle is unique in MyHC composition, expressing MyHC-α cardiac and MyHC-fetal isoforms as well as hitherto unrecognized potential spliced isoforms of MyHC-fetal and MyHC-IIx. Differences in masseter MyHC expression between young adult and elderly suggest a shift from childhood to adulthood towards more fast contractile properties. Differences between masseter and biceps are proposed to reflect diverse evolutionary and developmental origins and confirm that the masseter and biceps present separate allotypes of muscle.








Similar content being viewed by others
References
Arnostova P, Jedelsky PL, Soukup T, Zurmanova J (2011) Electrophoretic mobility of cardiac myosin heavy chain isoforms revisited: application of MALDI TOF/TOF analysis. J Biomed Biotechnol 2011:634253
Berg JS, Powell BC, Cheney RE (2001) A millennial myosin census. Mol Biol Cell 12:780–794
Bredman JJ, Wessels A, Weijs WA, Korfage JA, Soffers CA, Mooman AF (1991) Demonstration of “cardiac-specific” myosin heavy chain in masticatory muscles of human and rabbit. Histochem J 23:160–170
Butler-Browne G, Eriksson P-O, Laurent C, Thornell L-E (1988) Adult human masseter muscle fibres express myosin isozymes characteristic of development. Muscle Nerve 11:610–620
Cho M, Webster SG, Blau HM (1993) Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol 121:795–810
Desjardins PR, Burkman JM, Shrager JB, Allmond LA, Stedman HH (2002) Evolutionary implications of three novel members of the human sarcomeric myosin heavy chain gene family. Mol Biol Evol 19:375–393
Dubowitz V (2007) Muscle biopsy: a practical approach, vol 3. Bailliére Tindall, London, pp 47–74
Ecob-Prince M, Hill M, Brown W (1989) Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci 91:71–78
Engel A, Franzini-Armstrong C (2004) Myology: basic and Clinical, New York
Eriksson P-O (1982) Muscle-fibre composition of the human mandibular locomotor system: enzyme-histochemical and morphological characteristics of functionally different parts. Swed Dent J Suppl 12(Suppl):1–44
Eriksson P-O, Thornell L-E (1983) Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol 28:781–795
Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E (2009) Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16:822–832
Harzer W, Maricic N, Gedrange T, Lewis M, Hunt N (2010) Molecular diagnosis in orthodontics, facial, and orthognathic surgery: implications for treatment progress and relapse. Semin Orthod 16:118–127
Herron TJ, McDonald KS (2002) Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 90:1150–1152
Hoh JF (2002) Superfast or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol 205:2203–2210
Hoh JF (2005) Laryngeal muscle fibre types. Acta Physiol Scand 183:133–149
Hoh JF, Hughes S, Kang LHD, Rughani A, Qin H (1993) The biology of cat jaw-closing muscle cells. J Comput-Assist Microsc 5:65–70
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158:183–199
Kang L, Hughes S, Pettigrew J, Hoh J (1994) Jaw-specific myosin heavy chain gene expression in sheep, dog monkey, flying fox and microbat jaw-closing muscles. Basic Appl Myol 4:381–392
Karsch-Mizrachi I, Travis M, Blau H, Leinwand LA (1989) Expression and DNA sequence analysis of a human embryonic skeletal muscle myosin heavy chain gene. Nucleic Acids Res 17:6167–6179
Kjellgren D, Thornell L-E, Andersen J, Pedrosa-Domellöf F (2003) Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 44:1419–1425
Léger JJ (1985) Institut National de la Santé et de la Recherche Médical, Unité 249, Montpellier, France
Liu J-X, Eriksson P-O, Thornell L-E, Pedrosa-Domellöf F (2002) Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem 50:171–183
Lucas CA, Hoh JF (2003) Distribution of developmental myosin heavy chains in adult rabbit extraocular muscle: identification of a novel embryonic isoform absent in fetal limb. Invest Ophthalmol Vis Sci 44:2450–2456
Mahdavi V, Chambers AP, Nadal-Ginard B (1984) Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci USA 81:2626–2630
McCollum MA, Sherwood CC, Vinyard CJ, Lovejoy CO, Schachat F (2006) Of muscle-bound crania and human brain evolution: the story behind the MYH16 headlines. J Hum Evol 50:232–236
Monemi M, Eriksson P-O, Eriksson A, Thornell L-E (1998) Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci 154:35–48
Monemi M, Eriksson P-O, Kadi F, Butler-Browne GS, Thornell L-E (1999) Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil 20:351–361
Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235:1194–1218
Österlund C, Thornell L-E, Eriksson P-O (2011) Differences in fibre type composition between human masseter and biceps muscles in young and adults reveal unique masseter fibre type growth pattern. Anat Rec (Hoboken) 294:1158–1169
Oukhai K, Maricic N, Schneider M, Harzer W, Tausche E (2011) Developmental myosin heavy chain mRNA in masseter after orthognathic surgery: a preliminary study. J Craniomaxillofac Surg 39:401–406
Pedrosa-Domellöf F, Eriksson P-O, Butler-Browne G, Thornell L-E (1992) Expression of alpha-cardiac myosin heavy chain in mammalian skeletal muscle. Experientia 48:491–494
Pette D, Staron RS (2000) Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50:500–509
Qin H, Hsu MK, Morris BJ, Hoh JF (2002) A distinct subclass of mammalian striated myosins: structure and molecular evolution of “superfast” or masticatory myosin heavy chain. J Mol Evol 55:544–552
Ringqvist M (1973) Histochemical enzyme profiles of fibres in human masseter muscles with special regard to fibres with intermediate myofibrillar ATPase reaction. J Neurol Sci 18:133–141
Rossi AC, Mammucari C, Argentini C, Reggiani C, Schiaffino S (2010) Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 588:353–364
Rowlerson A, Pope B, Murray R, Weeds A (1981) A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil 2:415–438
Sambasivan R, Kuratani S, Tajbakhsh S (2011) An eye on the head: the development and evolution of craniofacial muscles. Development 138:2401–2415
Sawchak JA, Leung B, Shafiq SA (1985) Characterization of a monoclonal antibody to myosin specific for mammalian and human type II muscle fibers. J Neurol Sci 69:247–254
Schiaffino S (2010) Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199:451–463
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205
Schmidt-Nielson K (1984) Scaling: why animal size is so important. Cambridge University Press, Cambridge
Sewry CA, Uziyel Y, Torelli S, Buchanan S, Sorokin L, Cohen J, Watt DJ (1998) Differential labelling of laminin alpha 2 in muscle and neural tissue of dy/dy mice: are there isoforms of the laminin alpha 2 chain? Neuropathol Appl Neurobiol 24:66–72
Silberstein L, Webster SG, Travis M, Blau HM (1986) Developmental progression of myosin gene expression in cultured muscle cells. Cell 46:1075–1081
Smerdu V, Soukup T (2008) Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52:179–190
Soussi-Yanicostas N, Barbet J, Laurent-Winter C, Barton P, Butler-Browne GS (1990) Transition of myosin isozymes during development of human masseter muscle: persistence of developmental isoforms during postnatal stage. Development 108:239–249
Stål P, Eriksson P-O, Schiaffino S, Butler-Browne GS, Thornell L-E (1994) Differences in myosin composition between human orofacial, masticatory and limb muscles: enzyme-, immunohist- and biochemical-studies. J Muscle Res Cell Motil 15:517–534
Stedman HH, Kozyak BW, Nelson A, Thesier DM, Su LT, Low DW, Bridges CR, Shrager JB, Minugh-Purvis N, Mitchell MA (2004) Myosin gene mutation correlates with anatomical changes in the human lineage. Nature 428:415–418
Sternberger LA (1979) The unlabeled antibody (PAP) method, introduction. J Histochem Cytochem 27:1657
Swynghedauw B (1986) Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66:710–771
Talmadge RJ, Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75:2337–2340
Thornell L-E, Billeter R, Eriksson P-O, Ringqvist M (1984) Heterogenous distribution of myosin in human masticatory muscle fibres as shown by immunocytochemistry. Archs oral Biol 29:1–5
Thornell L-E, Grove B, Pedrosa F, Butler-Browne G, Dhoot G, Fischman D (1989) Expression of slow tonic myosin in muscle spindle fibres early in mammalian development. In: Stockdale F, Kedes I (eds) Molecular biology of muscle development. Alan R Liss, New York, pp 471–480
Walro JM, Kucera J (1999) Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci 22:180–184
Weiss A, Schiaffino S, Leinwand LA (1999) Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functioanl diversity. J Mol Biol 290:61–75
Acknowledgments
The authors wish to thank Mrs. Inga Johansson for excellent technical assistance and associate professor Albert Crenshaw for English revision and valuable comments. This work was supported by grants from the Department of Odontology, Umeå University, Västerbotten County Council and the Swedish Dental Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Österlund, C., Lindström, M., Thornell, LE. et al. Remarkable heterogeneity in myosin heavy-chain composition of the human young masseter compared with young biceps brachii. Histochem Cell Biol 138, 669–682 (2012). https://doi.org/10.1007/s00418-012-0985-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-0985-5