Abstract
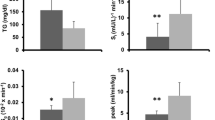
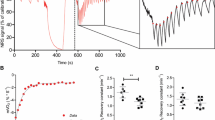
After spinal cord injury (SCI) skeletal muscle decreases in size, increases in intramuscular fat, and has potential declines in mitochondrial function. Reduced mitochondrial function has been linked to the development of metabolic disease. The aim of this study was to measure mitochondrial function in persons with SCI using near-infrared spectroscopy (NIRS). Oxygen consumption of the vastus lateralis muscle was measured with NIRS during repeated short-duration arterial occlusions in nine able-bodied (AB) and nine persons with motor complete SCI. Skeletal muscle oxidative capacity (V max) was evaluated with two approaches: (1) rate constant of the recovery of oxygen consumption after exercise and (2) extrapolated maximum oxygen consumption from a progressive work test. V max as indicated by the rate constant (k) from the recovery kinetics test was lower in SCI compared with AB participants (k: SCI 0.7 ± 0.3 vs. AB 1.9 ± 0.4 min−1; p < 0.001). Time constants were SCI 91.9 ± 37.8 vs. AB 33.6 ± 8.3 s. V max from the progressive work test approached a significant difference between groups (SCI 5.1 ± 2.9 vs. AB 9.8 ± 5.5 % Hb-Mb/s; p = 0.06). NIRS measurements of V max suggest a deficit of 50–60 % in participants with SCI compared with AB controls, consistent with previous studies using 31P-MRS and muscle biopsies. NIRS measurements can assess mitochondrial capacity in people with SCI and potentially other injured/diseased populations.






Similar content being viewed by others
Abbreviations
- AB:
-
Able-bodied
- AIS:
-
American Spinal Injury Association Impairment Scale
- ATP:
-
Adenosine triphosphate
- ATT:
-
Adipose tissue thickness
- MRS:
-
Magnetic Resonance Spectroscopy
- mVO2 :
-
Skeletal muscle oxygen consumption
- NIRS:
-
Near-infrared spectroscopy
- PCr:
-
Phosphocreatine
- SCI:
-
Spinal cord injury
- SDH:
-
Succinate dehydrogenase
- V max :
-
Skeletal muscle oxidative capacity
- 31P-MRS:
-
31-Phosphorous magnetic resonance spectroscopy
References
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Investig 119(3):573–581. doi:10.1172/jci37048
Argov Z, Bank W, Maris J, Chance B (1987) Bioenergetic heterogeneity of human mitochondrial myopathies: phosphorus magnetic resonance spectroscopy study. Neurology 37:257–262
Ballinger SW (2005) Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38(10):1278–1295. doi:10.1016/j.freeradbiomed.2005.02.014
Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI (2007) Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56(5):1376–1381. doi:10.2337/db06-0783
Bickel CS, Slade JM, Dudley GA (2004a) Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol 91(2–3):308–313. doi:10.1007/S00421-003-0973-5
Bickel CS, Slade JM, Dudley GA (2004b) Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol 91(2–3):308–313. doi:10.1007/s00421-003-0973-5
Brizendine JT, Ryan TE, Larson RD, McCully KK (2013) Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc 45(5):869–875
Buchheit M, Ufland P, Haydar B, Laursen PB, Ahmaidi S (2011) Reproducibility and sensitivity of muscle reoxygenation and oxygen uptake recovery kinetics following running exercise in the field. Clin Physiol Funct Imaging 31(5):337–346. doi:10.1111/j.1475-097X.2011.01020.x
Castro MJ, Apple DF Jr, Melton-Rogers S, Dudley GA (2000) Muscle fiber type-specific myofibrillar Ca(2+) ATPase activity after spinal cord injury. Muscle Nerve 23(1):119–121
Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, Smith D (1985) Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Nat Acad Sci 82:8384–8388
Chance B, Dait M, Zhang C, Hamaoka T, Hagerman F (1992) Recovery from exercise induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol 262:C766–C775
Chance B, Im J, Nioka Shoko, Kushmerick M (2006) Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed 19(7):904–926
Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R (1999) Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord 37(4):264–268
DeBlasi R, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A (1994) Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J Appl Physiol 76:1388–1393
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA (2004) Intramuscular fat and glucose tolerance after spinal cord injury: a cross-sectional study. Spinal Cord 42(12):711–716. doi:10.1038/sj.sc.31016523101652
Ferrari M, Binzoni T, Quaresima V (1997) Oxidative metabolism in muscle. Phil Trans R Soc Lond B 352:677–683
Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA (2009) Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 296(1):R161–R170. doi:90704.200810.1152/ajpregu.90704.2008
Hagströmer M, Oja P, Sjöström M (2006) The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9(6):755–762. doi:10.1079/PHN2005898
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biol Opt 12(6):062105
Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA (2012) Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37(1):88–99. doi:10.1139/h11-135
Lehninger A (1976) Biochemistry, 2nd edn. Worth Publishers, Inc., New York
Levy M, Kushnir T, Mizrahi J, Itzchak Y (1993) In vivo 31P NMR studies of paraplegics’ muscles activated by functional electrical stimulation. Magn Reson Med 29(1):53–58
Lutjemeier BJ, Ferreira LF, Poole DC, Townsend D, Barstow TJ (2008) Muscle microvascular hemoglobin concentration and oxygenation within the contraction-relaxation cycle. Respir Physiol Neurobiol 160(2):131–138. doi:S1569-9048(07)00243-110.1016/j.resp.2007.09.005
Mahoney E, Puetz TW, Dudley GA, McCully KK (2007) Low-frequency fatigue in individuals with spinal cord injury. J Spinal Cord Med 30(5):458–466
Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F (2010) Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: a parameter to be considered in a clinical setting? Angiology 61(6):530–536. doi:10.1177/0003319710362975
Martin TP, Stein RB, Hoeppner PH, Reid DC (1992) Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol 72(4):1401–1406
McCully KK, Boden BP, Tuchler M, Fountain MR, Chance B (1989) Wrist flexor muscles of elite rowers measured with magnetic resonance spectroscopy. J Appl Physiol 67(3):926–932
McCully KK, Fielding RA, Evans WJ, Leigh JS Jr, Posner JD (1993) Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75(2):813–819
McCully K, Mancini D, Levine S (1999) Nuclear magnetic resonance spectroscopy: its role in providing valuable insight into diverse clinical problems. Chest 116(5):1434–1441. doi:10.1378/chest.116.5.1434
McCully KK, Mulcahy TK, Ryan TE, Zhao Q (2011a) Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol 111(1):143–148. doi:10.1152/japplphysiol.00094.2011
McCully KK, Mulcahy TK, Ryan TE, Zhao Q (2011b) Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol 111(1):143–148. doi:10.1152/japplphysiol.00094.2011
Motobe M, Murase N, Osada T, Homma T, Uedo C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoskika A, Hamaoka T (2004) Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med 3(1):2
Nioka S, Kime R, Sunar U, Im J, Izzetoglu M, Zhang J, Alacam B, Chance B (2006) A novel method to measure regional muscle blood flow continuously using NIRS kinetics information. Dyn Med 5:5. doi:10.1186/1476-5918-5-5
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350(7):664–671. doi:10.1056/NEJMoa031314
Richardson RS, Noyszewski EA, Haseler LJ, Bluml S, Frank LR (2002) Evolving techniques for the investigation of muscle bioenergetics and oxygenation. Biochem Soc Trans 30(2):232–237
Ryan TE, Brizendine JT, McCully KK (2013) A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near infrared spectroscopy. J Appl Physiol 114(2):230–237. doi:10.1152/japplphysiol.01043.2012
Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK (2012) Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol 113(2):175–183. doi:10.1152/japplphysiol.00319.2012
Scott WB, Lee SC, Johnston TE, Binkley J, Binder-Macleod SA (2006) Contractile properties and the force-frequency relationship of the paralyzed human quadriceps femoris muscle. Phys Ther 86(6):788–799
Slade JM, Bickel CS, Dudley GA (2004) The effect of a repeat bout of exercise on muscle injury in persons with spinal cord injury. Eur J Appl Physiol 92(3):363–366. doi:10.1007/s00421-004-1103-8
Soden RJ, Walsh J, MIddleton JW, Craven ML, Rutkowski SB, Yeo JD (2000) Causes of death after spinal cord injury. Spinal Cord 38:604–610
Spinal Cord Injury: facts and figures at a glance (2013) Spinal cord injury model system. National Spinal Cord Injury Statistical Center, Birmingham, Alabama. https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202013.pdf. Accessed 16 May 2013
van Beekvelt M, Borghuis M, van Engelen B, Wevers R, Colier W (2001a) Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond) 101:21–28
van Beekvelt MCP, Shoemaker JK, Tschakovsky ME, Hopman MTE, Hughson R (2001b) Blood flow and muscle oxygen uptake at the onset and end of moderate and heavy dynamic forearm exercise. Am J Physiol Regul Integr Comp Physiol 280:R1741–R1747
Walter G, Vandenborne K, McCully KK, Leigh JS (1997) Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol 272(2 Pt 1):C525–C534
Wolf M, Ferrari M, Quaresima V (2007) Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt 12(6):062104. doi:10.1117/1.2804899
Acknowledgments
The authors would like to thank Jared Brizendine for his assistance in data collection. Funded in part by NIH R01 HD039676.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Erickson, M.L., Ryan, T.E., Young, HJ. et al. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol 113, 2275–2283 (2013). https://doi.org/10.1007/s00421-013-2657-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2657-0