Abstract
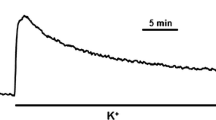
Strong, K+-induced contractions of rat aorta in Ca-free, Mg-free media were not accompanied by increased intracellular calcium concentration, [Ca2+]i, whereas such contractions in the presence of the divalent cations were correlated with rising [Ca2+]i as assessed by fura-2. At the same time, calcium channel blockers, a modulator of Ca2+-binding proteins, and a modulator of actin polymerization, inhibited all types of K+-induced contractions. Increasing the K+ in isotonic medium evoked a rise of 45Ca2+ binding to the plasma membrane of freshly isolated aortic cells. Although Ca2+-dependent events underlie the mechanism of K+-induced vascular contractions in both the presence and absence of Ca2+, in contrast to the view that [Ca2+]i is a key regulator of excitation-contraction coupling in smooth muscle, we suggest that the modulation of Mg2+-dependent Ca2+ binding, probably within/at the L-type calcium channel by K+, is a trigger for aortic contraction. This Ca2+ binding may then activate actin-myosin interaction.







Similar content being viewed by others
References
Altura BM, Altura BT (1985) New perspectives on the role of magnesium in pathophysiology in the cardiovascular system. II. Experimental aspects. Magnesium 4:245–271
Bakhramov A, Evans AM, Kozlowski RZ (1998) Differential effects of hypoxia on the intracellular Ca2+ concentration of myocytes isolated from different regions of the rat pulmonary arterial tree. Exp Physiol 83:337–347
Boström S-L, Ljung B, Mardth S, Forsen S, Thulin E (1981) Interaction of antihypertensive drug felodipine with calmodulin. Nature 292:777–778
Bruschi G, Bruschi ME, Regolisty G, Borghetti A (1988) Myoplasmic Ca2+-force relationship studied with fura-2 during stimulation of rat aortic smooth muscle. Am J Physiol 254:H840–H854
Daly MJ, Perry S, Mayer WG (1983) Calcium antagonists and calmodulin; Effect of verapamil, nifedipine and diltiazem. Eur J Pharmacol 90:103–108
Deng JT, Van Lierop JE, Sutherland C, Walsh MP (2001) Ca2+-independent smooth muscle contraction. J Biol Chem 276:16365–16373
Fay FS, Shlevin HH, Granger WC, Taylor SP (1979) Aequorin luminescence during activation of single isolated smooth muscle cells. Nature 280:506–508
Gerthoffer WT (1987) Disssociation of myosin phosphorylation and active tension during muscarinic stimulation. J Pharmacol Exp Ther 240:8–15
Godfraind T, Vanhoutte PM, Govoni S, Paoletti R (1985) Calcium entry blockers an tissue protection. Raven Press, New York
Goodman FR, Weiss GB (1971) Effect of lanthanum on45Ca movements on contractions induced by norepinephrine, histamine and potassium in vascular smooth muscle. J Pharmacol Exp Ther 177:415–425
Grynkiewicz G, Poenie M, Tsien R (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Hatem SN, Sweeten T, Vetter V, Morad M (1995) Evidence for the presence of Ca2+ channel-gated Ca2+ stores in neonatal human atrial myocytes. Am J Physiol 268:H195–H201
Himpens B, Matthijs G, Somlyo P (1989) Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol (Lond) 413: 489–503
Ito S, Kume H, Yamaki K, Katoh H, Honjo H, Kodama I, Hayashi H (2002) Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by rho-kinase in tracheal smooth muscle. Am J Respir 26:C491–C498
Itoh T, Suzuki H, Kuriyama H (1981) Effects of sodium depletion on contractions evoked in intact and skinned muscles of guinea-pig mesenteric artery. Jpn J Physiol 31:831–847
Kam KE, Stull J (2001) Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276:4527–4530
Karaki H, Weis GB (1981) Inhibitors of mitochondrial Ca2+ uptake dissociate potassium-induced tension responses from increased 45Ca retention in rabbit aortic smooth muscle. Blood Vessels 18:28–35
Karaki H, Ozaki H, Hori M, Misui-Saito M, Amano K-I, Harada K-I, Miayamoto S, Nakazawa H, Won K-J, Sato K (1997) Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49:157–230
Kargacin G, Fay FS (1987) Physiological and structural properties of saponin-skinned single smooth muscle cells. J Gen Physiol 90:49–73
Khoyi MA, Ishikawa T, Keef KD, Westfall DP (1996) Ca2+ induced inhibition of 45Ca2+ influx and Ca2+ current in smooth muscle of the rat vas deferens. Am J Physiol 270:C1468–C1477
Kojima Y, Tsunoda Y, Owyang C (1997) Adenosine 3′, 5′-cyclic monophosphate-stimulated Ca2+ efflux and acetylcoline release in ileal myenteric plexus are mediated by N-type Ca2+ channels: inhibition by the kappa opioid receptor agonist. J Pharmacol Exp Ther 282:403–409
Kravtsov GM, Kwan CY (1995) Regulation of vascular contraction by ionic matrix surfaces of smooth muscle cells: changes in SHR? Clin Exp Pharmacol Physiol [Suppl] 1:S237–S239
Kravtsov GM, Kwan CY (1995) A revisitation of the mechanism of action of KCl-induced vascular smooth muscle contraction: a key role of cation binding to the plasma membrane. Biol Signals 4:160–167
Kravtsov GM, Kwan CY (1996) Protein kinase plays no role in KCl-induced vascular contraction in Ca2+-free medium. Acta Pharmacol Sin 17:197–201
Kuo C-C, Hess P (1993) Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaechromocytoma cells. J Physiol (Lond) 466:683–706
Martell AE, Smith RM (1974) Critical stability constants, vol 1. Plenum Press, New York
Martell AE, Smith RM (1977) Critical stability constants, vol 3. Plenum Press, New York
Mita M, Yanagihara H, Hishiinuma S, Saito M and Walsh MP (2002) Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J 364:431–440
Mori M, Tsushima H (2000) Activation of Rho signaling contributes to lysophosphatic acid-induced contraction of intact ileal smooth muscle of guinea-pig. Can J Physiol Pharmacol 78:729–736
Movsesian MA, Swain AL, Adelstein RS (1984) Inhibition of turkey gizzard myosin light chain kinase activity by dihydropyridine calcium antagonists. Biochem Pharmacol 35:3759–3764
Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y (2000) Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol 278:C57–C65
Nakamura M, Sunagawa M, Kosugi T, Serekalis N (2000) Actin filaments disruption inhibits L-type Ca2+ channels current in cultured vascular muscle cells. Am J Physiol 279:C480–C487
Okada Y, Yanagisawa T, Taira N (1992) KCl-depolarization potentiates the Ca2+ sensitization by endothelin-1 in canine coronary artery. Jpn J Pharmacol 60:403–405
Peterson BZ, Catterall WA (1995) Calcium binding in the pore of L-type calcium channels modulates high affinity dihydropyridine binding. J Biol Chem 270:18201–18204
Pritchard K, Ashley CC (1987) Evidence for Na+/Ca2+ exchange in isolated smooth muscle cells: a fura-2 study. Pflugers Arch 410:401–407
Reig JA, Viniegra S, Ballesta JJ, Palmero M, Guitierrez LM (1993) Naphthalenesulfonamide derivatives ML9 and W7 inhibit catecholamine secretion in intact and permeabilized chromaffin cells. Neurochem Res 18:317–323
Sato K, Hori M, Ozaki H, Takano-Ohmuro H, Tsuchiya T, Sugi H, Karaki H (1992) Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J Pharmacol Exp Ther 261:497–505
Satoh M, Ishikawa T, Matsushima S, Naka, M, Hidaka H (1987) Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem 262:7796–7801
Somlyo AP, Somlyo AV (2000) Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol (Lond) 522:177–185
Tang C-M, Presser F, Morad M (1989) Amiloride selectively blocks the low threshold (T) calcium channel. Science 240:213–215
Triggle CR, Triggle DJ (1975) An analysis of the action of cations of the lanthanide series on the mechanical responses of guinea-pig ileal longitudinal muscle. J Physiol (Lond) 254:39–54
Ueno H (1985) Calcium mobilization in enzymically isolated single intact and skinned muscle cells of the porcine coronary artery. J Physiol (Lond) 363:103–117
Van Bremeen C, Wuytack F, Casteels R (1975) Stimulation of45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarization. Pflugers Arch 359:183–196
Acknowledgements
We thank Dr. F. Mo (The University of Hong Kong) for help in developing the fluorescent method for evaluation of [Ca2+]i in the rat aorta.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kravtsov, G.M., Bruce, I.C., Wong, T.M. et al. A new view of K+-induced contraction in rat aorta: the role of Ca2+ binding. Pflugers Arch - Eur J Physiol 446, 529–540 (2003). https://doi.org/10.1007/s00424-003-1096-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1096-x