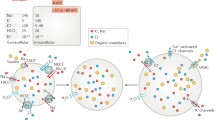
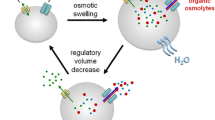
Abstract
Volume regulation is an essential property of any living cell and needs to be tightly controlled. While different types of K+ channels have been found to participate in the regulation of cell volume, the newly identified volume-regulated anion channel (VRAC) LRRC8 has been claimed to be essential for volume regulation. In unbiased genome-wide small interfering RNA (siRNA) screens, two independent studies identified LRRC8A/Swell1 as an essential component of VRAC, thus being indispensable for cellular volume regulation. We reanalyzed the role of LRRC8A for VRAC and regulatory volume decrease (RVD) in several cell types and under various conditions. While the role of LRRC8A for VRAC and its contribution to RVD is confirmed, we find that it is not essential for swelling-activated anion currents or cellular volume regulation, or apoptotic cell shrinkage. The contribution of LRRC8A is variable and largely depending on the cell type.










Similar content being viewed by others
References
Akita T, Okada Y (2011) Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589:3909–3927
Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K (2009) TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Galietta LJ, Haggie PM, Verkman AS (2001) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett 499:220–224
Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL (2003) cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125:1148–1163
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F (2013) Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int J Cancer 133:1631–1642
Jang Y and Oh U (2014) Anoctamin 1 in secretory epithelia. Cell Calcium 55:355-361
Jentsch TJ, Lutter D, Planells-Cases R, Ullrich F, and Voss FK (2015) VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch (in press)
Juul CA, Grubb S, Poulsen KA, Kyed T, Hashem N, Lambert IH, Larsen EH, Hoffmann EK (2014) Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflugers Arch 466:1899–1910
Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, Geha RS (2014) Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med 211:929–942
Lang F, Hoffmann EK (2012) Role of ion transport in control of apoptotic cell death. Compr Physiol 2:2037–2061
Milenkovic A, Brandl C, Milenkovic VM, Jendrike T, Sirianant L, Wanitchakool P, Zimmermann S, Reif CM, Horling F, Schrewe H, Strünker T, Alvarez L, Schreiber R, Kunzelmann K, Wetzel CH, and Weber BHF (2015) Bestrophin1 is the volume-regulated anion channel in mouse sperm and human retinal pigment epithelium. Proc.Natl.Acad.Sci U.S A. 112: E2630-E2639
Nilius B, Eggermont J, Voets T, Droogmans G (1996) Volume-activated Cl- channels. Gen Pharmacol 27:1131–1140
Nilius B, Prenen J, Voets T, Eggermont J, Droogmans G (1998) Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J Physiol 506:353–361
Okada Y (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24
Ousingsawat J, Wanitchakool P, Kmit A, Romao AM, Jantarajit W, Schreiber S, Kunzelmann K (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat Commun 6:6245
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94:419–459
Pedersen SF, Klausen TK, and Nilius B (2015) The identification of VRAC (volume regulated anion channel): an amazing Odyssey. Acta Physiol (Oxf). 213:268-281
Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, and Jentsch TJ (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 34:2993-3008
Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157:447–458
Sawada A, Takihara Y, Kim JY, Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera T, Ohta H, Ozono K, Hara J (2003) A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest 112:1707–1713
Sirianant L, Ousingsawat J, Wanitchakool P, Schreiber R, and Kunzelmann K (2016) Cellular volume regulation by anoctamin 6: Ca2+, phospholipase A2, osmosensing. Pflügers Arch 468:335-349
Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M (2014) Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J 28:2238–2248
Valverde MA, O'Brien JA, Sepulveda FV, Ratcliff R, Evans MJ, Colledge WH (1993) Inactivation of the murine cftr gene abolishes cAMP-mediated but not Ca2+-mediated secretagogue-induced volume decrease in small-intestinal crypts. Pflugers Arch 425:434–438
Valverde MA, O'Briens JA, Sepulveda FV, Ratcliff RA, Evans MJ, Colledge WH (1995) Impaired cell volume regulation in intestinal crypt epithelia of cystic fibrosis. Proc Natl Acad Sci 92:9038–9041
Valverde MA, Vazquez E, Munoz FJ, Nobles M, Delaney SJ, Wainwright BJ, Colledge WH, Sheppard DN (2001) Murine CFTR channel and its role in regulatory volume decrease of small intestine crypts. Cell Physiol Biochem 10:321–328
Vazquez E, Nobles M, Valverde MA (2001) Defective regulatory volume decrease in human cystic fibrosis tracheal cells because of altered regulation of intermediate conductance Ca2+-dependent potassium channels. Proc Natl Acad Sci U S A 98:5329–5334
Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344:634–638
Acknowledgments
This study was supported by DFG SFB699-A7/A12, DFG KU756/12-1, and Volkswagenstiftung AZ 87 499. HeLa cells stably expressing halide-sensitive YFP-H148Q/I152L were generously provided by Prof. Dr. M. Amaral, University of Lisbon, Portugal. HCT-wt (LRRC8A+/+) and HCT-LRRC8A−/− cells were from Dr. Voss/Prof. Dr. Jentsch (FMP, Berlin). pIRES2 LRRC8A-T44C was a generous gift from Dr. Zhaozhu Qiu (The Scripps Research Institute, La Jolla, USA). The excellent technical assistance by Mss. B. Wild, P. Seeberger, and E. Tartler is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirianant, L., Wanitchakool, P., Ousingsawat, J. et al. Non-essential contribution of LRRC8A to volume regulation. Pflugers Arch - Eur J Physiol 468, 805–816 (2016). https://doi.org/10.1007/s00424-016-1789-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1789-6